Understanding And Coping With Struvite/Vivianite Formation In WWTPs

Based on wastewater properties, various types of mineral buildup can affect wastewater treatment plant (WWTP) piping. With their impacts on plant energy efficiency and potential restriction of throughput capacity, keeping pipes free-flowing is an important aspect of WWTP operations. Here’s guidance for coping with two common problems associated with phosphorus concentrations in wastewater — struvite and vivianite.
The Source Of The Problem
Both struvite and vivianite can incur similar problems in WWTPs — capacity-reducing scaling and labor-intensive removal issues. The specific mineral formation depends on the amount of iron or magnesium present in the wastewater flow. Struvite and vivianite accumulation can be a common problem in anaerobic digesters. The buildup of mineral deposits on the inner walls of piping (Figure 1) can narrow the cross section of flow within the pipe, leading to reduced treatment capacity and higher energy costs for moving water through the wastewater treatment process.

Photo by Rob Baur of Clean Water Services
Figure 1. Struvite and vivianite buildup on pipe walls can drastically reduce flow efficiency in WWTPs.
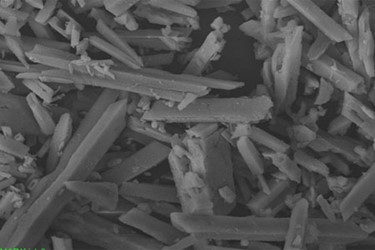
- Struvite comprises magnesium (9.9 percent), phosphorus (12.6 percent), hydrogen (6.6 percent), nitrogen (5.7 percent), and oxygen (65.2 percent) (Figure 2). It is formed as white, yellow-tinged, or brown-tinged scale when ammonia, phosphate, and magnesium are readily available after anaerobic digestion. It is a more prevalent problem at WWTPs using biological phosphorus removal (BPR), because the BPR process can double or triple the volume of phosphorus in the digestion process.

Photo by Rob Baur of Clean Water Services
Figure 2. Fresh struvite is clear, but it can easily take on other hues as a result of oxidization.
- Vivianite is a combination of iron (33.5 percent), phosphorus (12.3 percent), hydrogen (3.2 percent), and oxygen (51 percent) — all elements found in wastewater. It is formed when anaerobic digestion releases phosphate and changes ferric iron to ferrous iron, then the ferrous iron precipitates with sulfide and phosphate to form vivianite. In its initial formation, vivianite starts out in a colorless form but ends up as deep blue to deep blue-green or blue-gray crystals.
Because their formations are driven by the concentration of core elements, increased concentration of iron (in ferrous form) or magnesium will dictate which mineral will be formed. Both struvite (Figure 3) and vivianite formation are typically stimulated by rising pH levels that can occur in anaerobic digester effluent. Other factors can include temperature and the presence or conditions of ions in the water. Whether the minerals precipitate on interior piping surfaces or on WWTP equipment depends on the nature of the water quality. With high solids, suspended in the solution, precipitation will tend to take place in the suspended phase. With low solids, precipitation will tend to settle on hard surfaces.
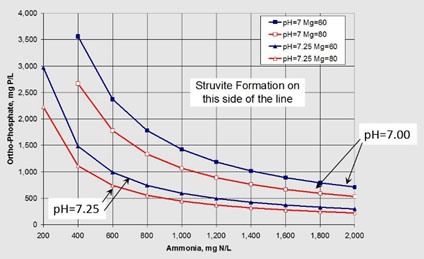
Chart provided courtesy of Bruce Johnson, PE, and Jacobs
Figure 3. At higher pH levels, the precipitation of struvite can take place with smaller concentrations of ammonia and orthophosphate.
The International Water Association (IWA) Task Group On Generalized Physicochemical Framework is in the process of developing a framework that can be used to understand and predict physicochemical reactions in wastewater operations. It could be useful for helping WWTP operators better appreciate the precipitation chemistry related to struvite and vivianite formation.
Choices For Struvite and Vivianite Prevention And Remediation
- Chemical Control Methods. Various chemical additives can be used to disrupt the process of struvite/vivianite formation and/or to encourage the breakdown of accumulations already built up on various surfaces within wastewater treatment process piping or equipment. They depend on creating specific chemical reactions to bind the building block elements of struvite/vivianite before they form or to break them down once they are established within the system.
- Non-Chemical Control Methods. Where chemical control methods are undesirable, several alternatives for preventing or removing struvite/vivianite buildup are still available:
- Dilution. Reducing the concentration of magnesium, ammonia, phosphorus, or iron in the wastewater flow can be used to discourage the buildup of struvite and vivianite on pipe and tank interiors or process equipment.
- Electric Pulses. An emerging technology for wastewater applications is being used to fight various types of scaling in water pipes and wastewater treatment plants. It is based on sending pulses of electrical energy through a pipeline to encourage the formation of struvite crystals suspended in the water flow instead of struvite scale forming on the inside surface of pipes or other equipment.
- Recycling. Yet another approach to struvite removal is to help it crystalize into small particles that can be removed from the wastewater flow and recycled as fertilizer for turf and agricultural applications.
More On The Phosphorus-Struvite-Vivianite Connection
Learn about a non-destructive thermal imaging method for identifying struvite accumulation in digester piping systems through this presentation available from the Texas Association of Clean Water Agencies. Gain a broader understanding of phosphorus removal, with a special reference to struvite, from this study guide from the Wisconsin Department of Natural Resources. Benefit from the experience of the Madison Metropolitan Sewerage District harvesting struvite for use as an agricultural fertilizer, as outlined in this case study and educational presentation. Finally, for a more in-depth review of phosphorus transformations in WWTPs, with some insights on the economic potential of struvite recovery, delve into this research document.
