A Guide To PFAS Treatment In Drinking Water
PFAS (per- and polyfluoroalkyl substances) are a large family of over 10,000 widely used synthetic chemicals that repel water, oil, stains, grease, and heat. However, they do not break down naturally, contaminating water, soil, and air, and are linked to serious health problems.
In the US, the EPA recently released the first enforceable national standards for PFAS removal and destruction, including guidelines for upgrading water treatment systems. Although necessary, these upgrades carry a high price tag for local water utilities and their ratepayers. This comprehensive guide explores the detection, removal, destruction technologies, and community engagement around PFAS treatment.
Table Of Contents:
- Why Are PFAS A Public Health Crisis?
- Understanding PFAS
- Health And Environmental Concerns Associated With PFAS
- PFAS Regulations And Guidelines
- PFAS Treatment Costs
- PFAS Detection And Monitoring
- PFAS Treatment Technologies
- PFAS Treatment System Design
- Operation And Maintenance Of PFAS Removal Systems
- PFAS Destruction Technologies
- Engaging With The Community About PFAS
- Future Outlook For PFAS Removal And Discharge
- Final Thoughts
- Frequently Asked Questions (FAQs)
- References
Why Are PFAS A Public Health Crisis?
PFAS were invented in the 1940s and have been widely used across multiple industries in the decades following their introduction. Due to their everyday use in consumer products and industrial applications, PFAS substances have infected water supplies worldwide. PFAS can be found in water sources across the US, and a US Geological Survey (USGS) study estimated that over 45% of US tap water contains one or more types of PFAS, affecting millions of people.
Health Risks Associated With PFAS Exposure
PFAS can interfere with normal bodily processes and bioaccumulate over time, creating risks that increase with exposure. Certain PFAS like PFOA and PFOS are associated with a variety of diseases and conditions, including cancer, reproductive issues, thyroid dysfunction, organ damage, developmental delays, and impaired immune response. Given their prevalence, they have become a global health issue.
Growing Regulatory Measures And Need For PFAS Removal From Drinking Water
Governments and regulatory bodies are increasingly restricting and phasing out PFAS, particularly the most studied and detrimental compounds like PFOA and PFOS. However, the large number of PFAS compounds makes regulation challenging. Efforts are underway to develop PFAS-free alternatives and clean up PFAS contamination, but the extreme persistence of these "forever chemicals" makes them difficult to remove from the environment once released.
Understanding PFAS
PFAS are synthetic chemicals with fluorine-carbon bonds that resist heat, water, and oil. They're used in a wide variety of industrial and commercial products, including non-stick cookware, waterproof fabrics, and firefighting foam.
Definition And Types Of PFAS
Although the term PFAS encompasses thousands of chemicals, they each possess at least one fully fluorinated carbon atom. They generally include compounds with a perfluoroalkyl moiety (C-F2-C-) or a perfluoroalkyl ether moiety (C-F2-O-C-F2-). However, regulatory definitions often need to be more precise, such as requiring a minimum number of fully fluorinated carbons (e.g., three or more).
Types of PFAS include:
-
Perfluoroalkyl substances (PFAAs): Fully fluorinated alkyl substances, such as PFOA and PFOS. These are considered the most concerning PFAS
-
Polyfluoroalkyl substances (PFAS precursors): Partially fluorinated alkyl substances that can transform into PFAAs in the environment
-
Fluoropolymers: Polymers with a carbon-only backbone and fluorine atoms directly attached, such as PTFE and PVDF
-
Side-chain fluorinated polymers: Polymers with a non-fluorinated carbon backbone and polyfluoroalkyl side chains
-
Perfluoropolyethers (PFPEs): Fluorinated polymers with carbon-oxygen backbones
PFAS Contamination In Drinking Water
Given the prevalence of PFAS in the commercial and industrial sectors, it’s not surprising that drinking water contamination is widespread in the U.S. and abroad.
PFAS are released into the environment through various industrial and consumer products, including firefighting foams, non-stick cookware, stain-resistant textiles, and food packaging. These chemicals can contaminate surface water and groundwater sources used for drinking water, especially near sites of PFAS manufacturing or use. Until recently, inadequate monitoring and management of PFAS in the environment led to ongoing source pollution and incomplete removal of these "forever chemicals."
Health And Environmental Concerns Associated With PFAS
PFAS have been detected in drinking water, surface water, groundwater, and even remote environments like the Arctic, creating widespread contamination that harms humans, animals, and the global environment. Due to their persistence, cleanup and remediation of PFAS contamination are highly challenging.
What Health Conditions Are Associated With PFAS Exposure?
PFAS resist degradation, which allows them to accumulate over time in organisms. Once in the body, PFAS typically gravitate to protein-rich areas, particularly blood and liver tissues. Many PFAS compounds have long half-lives in organisms and are not filtered out through the renal system like other foreign substances.
Due to their high prevalence, bioaccumulation, and persistence, PFAS pose serious health risks that increase with exposure, including:
-
Reproductive effects: decreased fertility and increased risk of high blood pressure in pregnant women
-
Developmental effects: low birth weight, accelerated puberty, behavioral changes in children
-
Increased cancer risk: prostate, kidney, and testicular cancers
-
Immune system impacts: reduced ability to fight infections, decreased vaccine response
-
Hormonal disruption: interference with the body's natural hormones
-
Metabolic diseases: increased cholesterol and obesity risk
-
Liver disorders: enzyme changes and non-alcoholic liver disease
-
Thyroid effects: dysfunction and increased thyroid disease
How Do PFAS Bioaccumulate?
PFAS bioaccumulate through complex mechanisms in living organisms. Phospholipid partitioning is the primary driver of bioaccumulation for PFAS with 8 or more perfluorinated carbons, allowing these chemicals to integrate into cellular membranes. For shorter-chain PFAAs, protein binding plays a crucial role, as these compounds can attach to specific proteins in blood plasma and tissues.
PFAS accumulation typically occurs when organisms ingest contaminated food, water, or sediment. This leads to biomagnification, where PFAS concentrations increase at higher trophic levels in the food web. In some cases, PFAS have been found at levels 5000 times higher in biota than in the surrounding water.
Notably, bioaccumulation potential varies among different PFAS compounds. Generally, longer-chain PFAS show higher bioaccumulation factors compared to their shorter-chain counterparts. This variation in bioaccumulation potential is a key consideration in assessing the environmental and health impacts of different PFAS.
How Do PFAS Affect Ecosystems And The Food Chain?
PFAS do not easily break down in the environment, contaminating water, soil, and air and leading to widespread environmental exposure. They accumulate in plants and animals, transferring through food chains and posing threats to entire ecosystems. The effects on wildlife are wide-ranging, disrupting hormones, reducing reproduction, and causing diseases.
PFAS damage is remarkably widespread. In soil, these chemicals can alter pH and structure, which harms soil organisms, a vital rung on the food chain. Water bodies are likewise contaminated with PFAS, harming water ecosystems. Plants in affected areas take in these chemicals, transferring them to animals and humans. Even in the air, PFAS can travel through atmospheric processes and deposit on soil surfaces.
As PFAS move from microorganisms and plants to animals, their concentrations and harm increase. Bioaccumulation and biomagnification are especially damaging to animals, accumulating in protein-rich areas like blood and liver tissues. Wild animals are exposed by consuming plants or animals, and predators are particularly vulnerable to bioaccumulation. Even domesticated animals, such as livestock and poultry, face high risks from PFAS exposure, which damages our food sources.
PFAS Regulations And Guidelines
In response to the dangers that PFAS pose, regulatory bodies such as the EPA are creating new guidelines to reduce contamination, safeguard ecosystems, and improve human health.
EPA’s PFAS Drinking Water Regulations: Background
Regulations and guidelines regarding PFAS changed dramatically in 2023 and 2024. On April 10, 2024, the Biden-Harris administration issued groundbreaking legislation to protect Americans from PFAS. This rule is the culmination of the EPA's PFAS Roadmap strategy launched in 2021 as a three-year plan to combat PFAS contamination. Critical steps in the formation of this rule included:
-
April 2023: the EPA issued an Advance Notice of Proposed Rulemaking to gather public input on potential future PFAS regulations under the Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA), also known as Superfund.
-
June 2023: the EPA released a framework for addressing new uses of PFAS under TSCA to ensure they pose no harm before entering commerce.
-
August 2023: the EPA finalized its National Enforcement and Compliance Initiatives for 2024-2027, including "Addressing Exposure to PFAS" to implement the EPA's PFAS Strategic Roadmap.
-
October 2023: the EPA published a final rule under the Toxic Substances Control Act (TSCA) that requires all manufacturers (including importers) to report PFAS production volumes, disposal, exposures, and hazards. This provides the EPA, its partners, and the public with the largest-ever dataset on PFAS manufactured and used in the U.S.
-
January 2024: the EPA finalized a rule establishing the first-ever nationwide, legally enforceable drinking water standards to protect communities from PFAS in their drinking water. This rule sets Maximum Contaminant Levels (MCLs) for PFOA and PFOS, the two most well-studied PFAS compounds. The EPA is also conducting the most comprehensive monitoring effort for 29 PFAS in public water systems through the Unregulated Contaminant Monitoring Rule 5 (UCMR 5).
Key Standards In The 2024 PFAS Rule
The Biden-Harris administration's goal is to protect 100 million Americans from PFAS. Therefore, the guidelines outlined in the new 2024 rule are designed to be strict and far-reaching to combat this growing health crisis aggressively:
-
Maximum contaminant levels (MCLs): The enforceable MCL for PFOA and PFOS is set at 4.0 parts per trillion (ppt) individually. For PFNA, PFHxS, and GenX chemicals, the MCL is set at 10 ppt.
-
Health-based goals: The non-enforceable, health-based goal for PFOA and PFOS is set at zero.
-
Mixture standard: A limit is also set for any combination of four PFAS: PFNA, PFHxS, PFBS, and GenX chemicals.
-
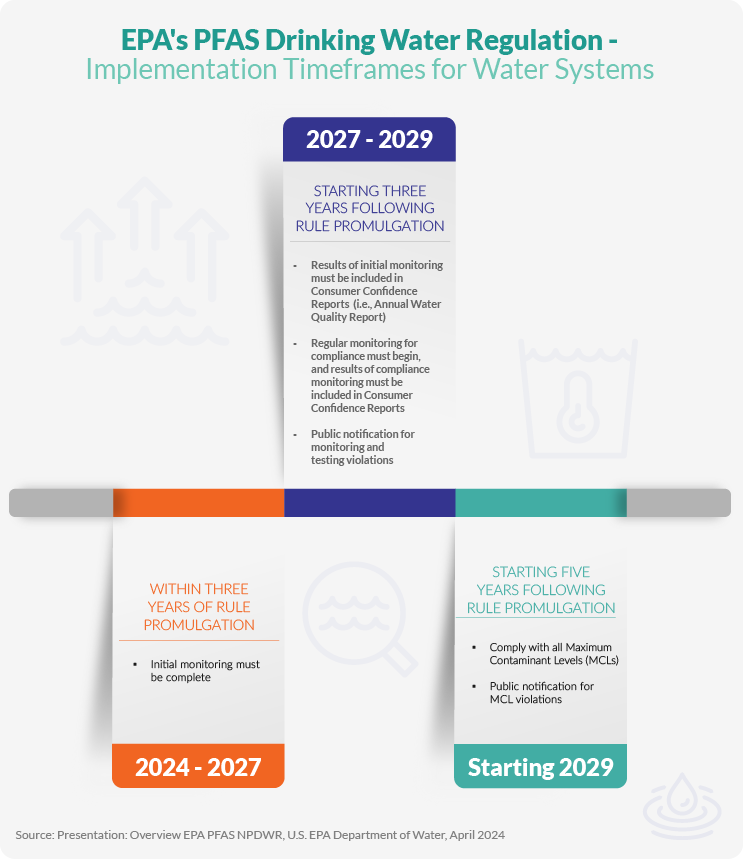
Compliance timeline: Public water systems have three years to complete initial monitoring for these PFAS. Where PFAS levels exceed the standards, systems must implement solutions to reduce PFAS within five years.
-
Public notification: Water utilities must inform the public of the PFAS levels measured in their drinking water.
Kyle Thompson, National PFAS Lead at Carollo Engineers, discusses the impact of the EPA’s April 2024 PFAS rule with Travis Kennedy and Kevin Westerling on the Water Online Show.
CERCLA (Superfund) Designation Implications
The CERCLA hazardous substance designation for PFOA and PFOS under the Superfund law represents an important step in the regulatory framework and has far-reaching implications for the entire water industry as well as other sectors responsible for PFAS cleanup. This designation results in the following:
-
Expanded cleanup obligations: CERCLA designates PFOA and PFOS as hazardous substances, requiring parties responsible for PFAS contamination to conduct cleanup and remediation efforts. This will significantly expand the number of sites where PFAS cleanup is required, as CERCLA provides a framework for identifying, investigating, and remediating contaminated sites.
-
Increased reporting and disclosure requirements: The CERCLA hazardous substance designation triggers new reporting requirements for releases of PFOA and PFOS into the environment. This leads to more comprehensive data on PFAS contamination sources and locations, which will inform future regulatory actions.
-
Potential liability concerns: Designating PFOA and PFOS as CERCLA hazardous substances exposes a broader range of parties to potential liability for PFAS contamination, including manufacturers, users, and waste handlers. This will likely incentivize companies to reduce PFAS use and properly manage PFAS-containing waste to avoid cleanup costs and legal liabilities.
The CERCLA designation alone may not be sufficient to fully address the broader class of PFAS compounds, as it is limited to just two specific substances. Ongoing challenges remain in terms of identifying all PFAS sources, establishing appropriate cleanup standards, and securing adequate funding for remediation efforts.
Impact Of CERCLA Designation On Water Treatment Plants And Utilities
The CERCLA designation of PFOA and PFOS as hazardous substances carries significant implications for water treatment plants and utilities. While the EPA has stated it will not pursue enforcement actions against public water systems and wastewater treatment plants, these entities may still be liable under CERCLA, exposing them to legal and financial repercussions.
The possibility of third-party lawsuits is a primary concern. Water utilities may face contribution claims from PFAS manufacturers and other responsible parties, resulting in substantial litigation costs. Additionally, local governments and utilities could be held liable for cleanup costs of contaminated sites, even for lawful past activities. This financial burden could strain budgets and impact service quality or rates for consumers.
The CERCLA designation also introduces new operational challenges. Facilities may need to investigate and report PFOA and PFOS releases above the reportable quantity, increasing their administrative responsibilities. Additionally, previously closed sites may be reopened as part of the CERCLA 5-year review process, affecting utilities near these locations. For existing CERCLA sites nearing closure, this designation could delay timelines and increase remediation costs.
Fortunately, Congress is working to provide statutory protections for water utilities. Legislation like the Water Systems PFAS Liability Act aims to shield these essential services from undue legal and financial burdens. However, as the situation continues to evolve, water treatment plants and utilities must remain vigilant and adaptable to navigate this complex regulatory landscape.
CERCLA Compliance Timelines
Complying with CERCLA is a multi-stage process that can take years or even decades to complete, from identification to remediation. Actual timelines will vary based on site conditions and complexity, but a typical timeline entails:
- Preliminary assessment (PA): Initially, the Department of Defense reviews existing information and possibly conducts site reconnaissance to determine potential hazardous substance releases. This phase may last one to two years.
- Site inspection (SI): When necessary, this step follows the PA and gathers sampling data.
- National priorities list (NPL) listing: If appropriate, this follows PA/SI.
- Remedial investigation/feasibility study (RI/FS): This must begin within six months of NPL inclusion to determine the nature and extent of contamination.
- Interagency agreement (IAG): The agency head must enter into an IAG with EPA within 180 days after the RI/FS review.
- Remedial design/remedial action (RD/RA): After the RI/FS and IAG.
- Long-term operation and maintenance: After the completion of remedial actions.
PFAS Treatment Costs
Complying with these new rules comes with a multi-billion-dollar price tag. However, the new guidance allows utility providers to hold polluters accountable for the high costs of compliance, and numerous lawsuits have already been filed in several states around the country in response to the new guidelines.
Drinking Water And Wastewater Utility Costs
According to a report by the American Water Works Association (AWWA) and Black & Veatch, drinking water utilities must invest over $50 billion over the next 20 years to install and operate treatment technology to comply with new PFAS standards. This includes the costs of designing, constructing, and operating treatment systems to remove PFAS from drinking water supplies.
Regarding wastewater, a survey by the National Association of Clean Water Agencies (NACWA) suggests that operational costs for individual clean water utilities will increase by up to 60% due to new PFAS regulations. For example, a study from Minnesota estimated the total wastewater costs for that state to remove PFAS to be between $14 and $28 billion over 20 years. Extrapolating this nationally, wastewater utilities are facing billions of dollars per annum in additional costs.
Under CERCLA, a hazardous substance designation for PFOA and PFOS could add another $3.5 billion per year in disposal costs for the water sector. This designation aims to uphold a "polluter pays" principle, but water utilities fear they may be unfairly burdened with cleanup costs despite not being responsible for PFAS production. Therefore, companies and utilities will need to plan for these substantial expenses in the coming years carefully.
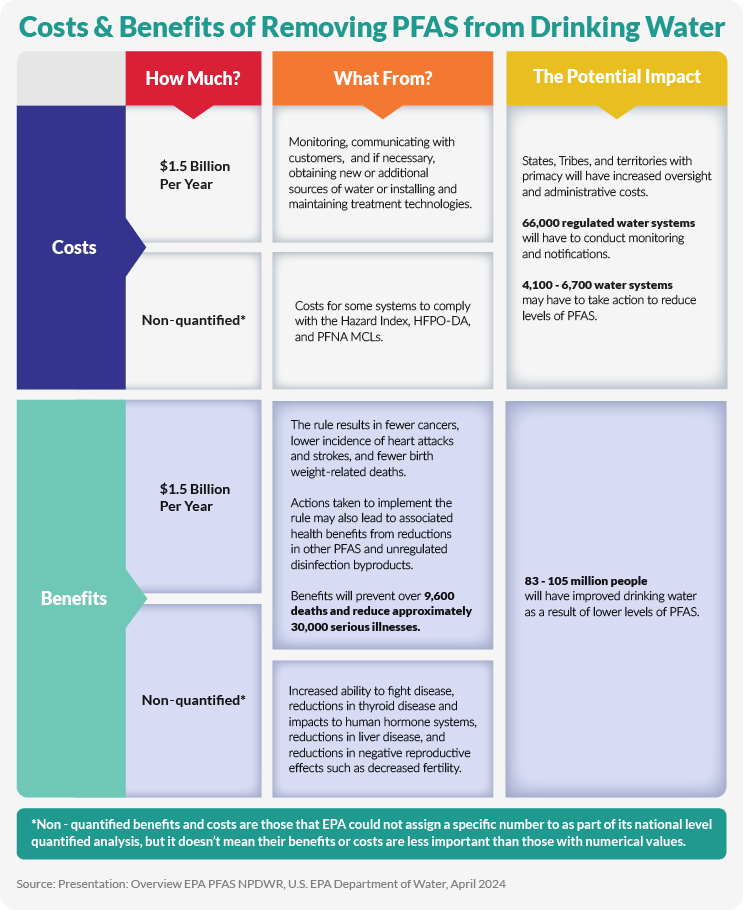
Financial Implications For Consumers
The high cost of compliance is expected to ultimately affect consumers as well. The AWWA estimates rate hikes ranging from $305 to $3,570 per household, with smaller communities facing the highest increases as fewer households share the total cost. Some utilities are already preparing for these expenses, with the Greater Augusta Utility District in Maine, for example, gearing up to spend $3 to $5 million on PFAS removal technology.
Lawsuits And Efforts To Shift Cost Burdens To Polluters
The costs of PFAS remediation have fallen on companies, utilities, and consumers, but multiple lawsuits and legal efforts are working to shift financial responsibility to the polluters who contaminated the water in the first place. For instance, the National Rural Water Association filed a class action lawsuit in 2020 on behalf of public water utilities and secured settlements totaling over $13 billion from major chemical companies like 3M, Corteva, DuPont, and Chemours.
In another example, a multidistrict litigation bellwether case regarding aqueous film-forming foam is also underway, encompassing hundreds of lawsuits against chemical manufacturers and other companies that contributed to PFAS contamination. Additionally, states and local governments have successfully sued some PFAS manufacturers for contaminating drinking water supplies, while water providers are increasingly filing individual lawsuits to recover cleanup and mitigation costs. These legal actions collectively seek to shift the financial burden of PFAS remediation from water utilities and taxpayers to the companies responsible for producing and using these chemicals.
PFAS Detection And Monitoring
Before cleanup can begin, water utilities must test for PFAS contamination and have strategies in place for continuous monitoring. The EPA has approved several methods for testing and monitoring water quality.
EPA-Approved Drinking Water Methods
The EPA has approved two primary methods for testing drinking water:
-
EPA Method 533: Determination of PFAS in Drinking Water by Isotope Dilution Anion Exchange SPE and LC/MS/MS
-
EPA Method 537.1: Determination of Selected PFAS in Drinking Water by SPE and LC/MS/MS
These methods are designed and validated for analyzing 29 PFAS compounds in drinking water samples and are the recommended techniques for utilities to comply with new PFAS regulations.
Importance Of Regular Monitoring
Regular monitoring allows operators to assess treatment efficacy, optimize processes, and ensure compliance with evolving regulations. Advanced analytical techniques like LC-MS/MS and GC-MS provide accurate PFAS concentration measurements before and after treatment.
Real-time monitoring enables prompt adjustments, while long-term monitoring tracks contamination trends and remediation effectiveness. Comprehensive monitoring programs validate treatment performance, demonstrate regulatory compliance, and protect public health.
Non-Potable Water And Environmental Media Methods
Methodologies for testing non-potable water and environmental media are still in development under EPA Draft Method 1633: Detection of PFAS in non-potable water, soil, sediment, and other ecological matrices using SPE and LC-MS/MS. This draft method is intended to expand PFAS analysis capabilities beyond just drinking water samples.
Emerging Detection Techniques
Scientists are working to develop more efficient methods for detecting PFAS contamination, including:
-
Fluorescent chemosensors: novel optical sensors that can detect and quantify PFAS in aqueous samples
-
3D-printed cone spray ionization (3D-PCSI) with mass spectrometry: an innovative technique for rapid, on-site PFAS detection
-
Optical fiber sensor networks: used to detect PFOA in water samples
These emerging techniques may provide faster, more portable, and potentially lower-cost alternatives to the standard LC-MS/MS methods. However, they have not yet been as extensively validated or approved for regulatory compliance.
PFAS Treatment Technologies
Water utilities have a variety of options for removing PFAS from drinking water to meet new compliance requirements.
Activated Carbon
Activated carbon filtration, in both granular (GAC) and powdered (PAC) forms, is the most studied and widely used treatment technology for removing PFAS from drinking water. GAC can be up to 100% effective at eliminating longer-chain PFAS like PFOA and PFOS, depending on factors like the type of carbon, flow rate, and presence of other contaminants. Shorter-chain PFAS like PFBS and PFBA do not adsorb as well onto activated carbon, resulting in earlier "breakthroughs" and the need for more frequent carbon replacement or regeneration.
Mechanism Of PFAS Adsorption
Activated carbon, in both GAC and PAC forms, adsorbs PFAS compounds at the interface between the liquid (water) and solid (carbon) phases. It is an effective adsorbent due to its highly porous structure, which provides a large surface area for contaminants like PFAS to adsorb onto.
-
Influence of PFAS chain length: Longer-chain PFAS like PFOA and PFOS are better adsorbed by activated carbon than shorter-chain PFAS like PFBA and PFPeA. This is due to the decreasing polarity of PFAS compounds as the carbon chain length increases, leading to stronger adsorption interactions with the carbon surface.
-
Adsorption capacity: The adsorption capacity of activated carbon for PFAS increases with chain length, as evidenced by higher Freundlich adsorption coefficients (KF) and lower Freundlich exponents (n). This means longer-chain PFAS can be adsorbed to a greater extent before the carbon becomes saturated.
-
Breakthrough behavior: Shorter-chain PFAS, like PFBA, exhibits earlier breakthroughs in GAC filters, meaning the carbon becomes saturated more quickly and requires more frequent replacement or regeneration. In contrast, longer-chain PFAS like PFOA can be effectively removed by GAC for a longer period before a breakthrough occurs.
GAC Filtration Process
GAC is typically used in a flow-through filter mode, where the water passes through a bed of granular activated carbon. The PFAS compounds adsorb onto the large surface area of the porous carbon material as the water flows through. Pretreatment to remove particulates and other contaminants is often required before the GAC filtration step to prevent fouling and maintain performance.
PAC Application
PAC can be added directly to the water and removed through filtration or clarification. However, it is less efficient and economical than GAC for PFAS removal, as it has a lower adsorption capacity and requires higher doses to achieve modest removal rates.
Additional Factors Influencing Activated Carbon Efficiency
Although activated carbon is a popular method for removing PFAS, utilities must consider specific operational factors when designing an activated carbon system.
-
GAC replacement or regeneration frequency is a critical operational factor, as the carbon's PFAS removal efficiency decreases over time. When designing GAC systems, utilities must also plan for soaking and backwash requirements, pH adjustment, arsenic content, and disinfectant. Disposal or incineration of the spent, PFAS-laden carbon is another crucial environmental consideration.
-
The type of activated carbon, including its surface charge, pore-size distribution, and surface area, can impact its PFAS adsorption capacity and efficiency. Positively charged activated carbons generally show higher adsorption capacities for PFAS compared to neutral or negatively charged carbons.
-
The competition from other organic compounds, such as total organic carbon (TOC), can reduce the available adsorption sites on the activated carbon and decrease its PFAS removal efficiency. Pretreatment to remove particulates and other contaminants is often required before the GAC filtration step to prevent fouling and maintain performance.
-
Operating conditions such as flow rate, bed depth, and contact time can affect the PFAS removal rates, with lower flow rates and deeper beds generally improving performance.
-
The disposal or incineration of the spent, PFAS-laden activated carbon is an important environmental consideration, as the PFAS compounds are not destroyed during the process.
-
Regeneration of the activated carbon can help extend its useful life, but the regenerated carbon may not be as effective as virgin carbon for PFAS removal.
Ion Exchange
Ion exchange (IX) is an effective technology for the remediation of PFAS-contaminated water, including surface water, groundwater, and wastewater. PFAS compounds, which are anionic, zwitterionic, and neutral, can be removed through adsorption and ion exchange mechanisms on specialized IX resins. The adsorption capacity of IX resins for PFAS can be up to 5 millimoles per gram (mmol/g), with factors like resin porosity, PFAS chain length, and competing ions influencing the adsorption process. This removal process entails diffusion, electrostatic interactions, and hydrophobic effects between the PFAS and the resin surface.
Application In Drinking Water Treatment
IX resins can be more effective than activated carbon at removing emerging short-chain PFAS that are not well adsorbed by carbon-based processes. IX can achieve PFOA and PFOS removal rates of 77-99%, with the highest rates for sulfonates and longer-chain PFAS. These systems can also be designed and optimized for specific PFAS compounds, allowing for higher capacity and more targeted removal compared to general adsorption processes.
Challenges And Considerations
Despite these advantages, other competing ions and organic matter in the water can impact the IX resin's PFAS removal efficiency and require pretreatment. Regeneration of the spent IX resin is another important consideration, as its eluent composition and reuse can affect its long-term performance. Disposal or incineration of the PFAS-laden IX waste stream is a further environmental concern that must be addressed. Finally, capital and operating costs of IX systems can be similar to or higher than activated carbon, depending on the specific resin and treatment requirements.
Membrane Filtration
Membrane filtration, including nanofiltration (NF) and reverse osmosis (RO), is an effective technology for removing PFAS from water. The membrane acts as a physical barrier, allowing water to pass while retaining PFAS and other contaminants. Membrane filtration can achieve removal rates of over 99% for PFOA and PFOS and 84-99% for other PFAS compounds. However, this type of filtration can be impacted by fouling, scaling, and high operating costs compared to other treatment methods like activated carbon and ion exchange.
Specific Membranes For PFAS Removal
Novel membrane materials have been developed to improve PFAS removal, such as fluorinated silane-functionalized aluminum oxide hydroxide membranes. Polymeric membranes modified with adsorbents like nanoparticles or graphene oxide can also enhance PFAS rejection. Ceramic membranes and nanoparticle-coated silica membranes have shown high PFAS removal efficiency, though they may experience irreversible changes to the membrane surface over time. Reactive electrochemical membranes and phosphorene nanocomposite membranes can not only remove PFAS but also degrade them through advanced oxidation processes.
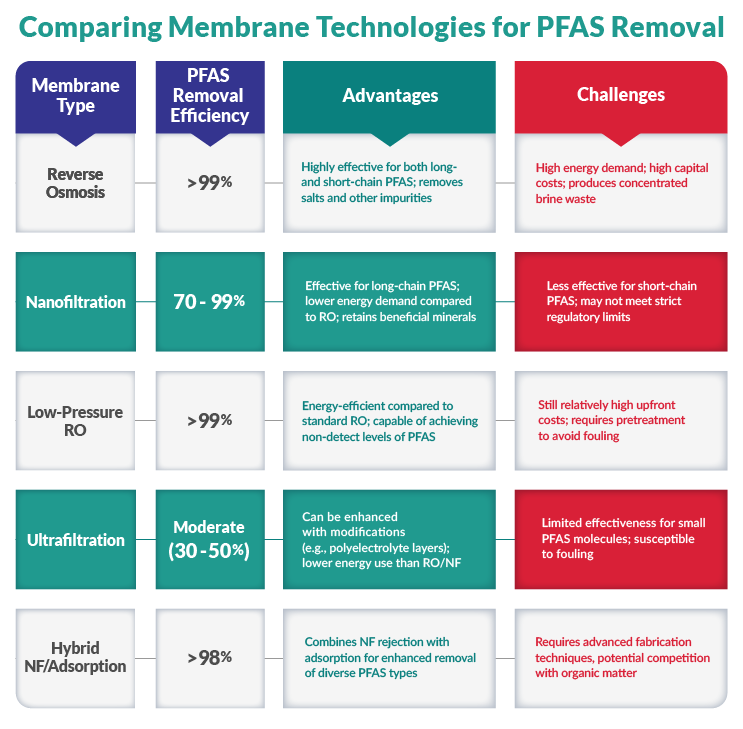
Performance And Limitations
Membrane filtration is generally more effective at removing longer-chain PFAS like PFOA and PFOS compared to shorter-chain PFAS, but the presence of other contaminants like organic matter and dissolved solids can impact membrane performance and lead to fouling. Membrane systems require high operating pressures, which increases energy consumption and costs, and the waste-concentrated stream also needs to be managed appropriately. While membrane filtration is a promising technology, ongoing research is focused on improving materials, reducing fouling, and lowering operating costs to make it more widely deployable for PFAS removal.
Advanced Oxidation Processes (AOPs)
AOPs offer another effective method for eradicating PFAS from drinking water. These highly reactive oxidizing species, such as hydroxyl radicals, degrade and transform PFAS compounds in water. Standard AOP technologies include UV, UV/H2O2, ozone, ozone/H2O2 (peroxone), and photocatalysis using materials like TiO2 and boron nitride. The goal of AOPs is to break down the solid carbon-fluorine bonds in PFAS, leading to defluorination and the formation of shorter-chain or more easily removable compounds.
Comparative Analysis Of AOPs
Studies show that different AOP technologies have varying effectiveness for PFAS removal, with some being more suitable for certain PFAS compounds than others. For example, ozone and UV/H2O2 can achieve high removal rates (up to 100%) for longer-chain PFAS like PFOA and PFOS while less effective for shorter-chain PFAS. Photocatalytic processes using materials like TiO2 and boron nitride have also demonstrated promising PFAS degradation capabilities. The optimal AOP approach often depends on factors like PFAS composition, water matrix, operating conditions, and cost-effectiveness.
Real-World Applications And Outcomes
While AOPs have shown potential for PFAS removal in lab and pilot-scale studies, their real-world application is not straightforward. Unfortunately, AOPs can transform non-detected or unknown longer-chain PFAS into detectable shorter-chain PFAS, leading to an overall increase in the total PFAS concentration. This "transformation" of PFAS compounds makes it difficult to accurately assess the effectiveness of AOPs for PFAS removal, highlighting the need for more comprehensive analytical methods. To address this, some studies suggest using adsorbable organic fluorine (AOF) analysis before and after AOP treatment as a more holistic approach to evaluating PFAS removal.
Comparing The Effectiveness, Advantages, And Limitations Of PFAS Removal Technologies
Activated carbon, ion exchange, membrane filtration, and AOP offer distinct advantages and limitations when used to remove PFAS from water. Key differences include:
Activated Carbon
- Effectiveness: Removes 80-90% of PFAS, especially effective for long-chain PFAS.
- Advantages: Low cost ($0.093-0.12/m³), can remove low concentrations (ng/L) from drinking water.
- Limitations: Less effective for short-chain PFAS, reduced efficiency with organic compounds present, energy-intensive regeneration.
Ion Exchange
- Effectiveness: Highly efficient for anionic and long-chain PFAS, even at ng/L concentrations.
- Advantages: Higher adsorption capacity than activated carbon, fast adsorption kinetics, operating cost of about 60% of activated carbon.
- Limitations: Less efficient with organic/inorganic matter present, limited removal of short-chain PFAS, expensive regeneration.
Membrane Filtration
- Effectiveness: RO removes >99% of PFAS, NF removes 70-99%.
- Advantages: RO is effective for both long- and short-chain PFAS, and NF retains beneficial minerals.
- Limitations: High energy demand and capital costs for RO, NF less effective for short-chain PFAS.
AOP
- Effectiveness: Ozone with iron-oxide catalyst and persulfate can reach 70% PFAS removal in tap water.
- Advantages: It has the potential to destroy rather than transfer PFAS and is effective for medium-chain PFAS (6-11 CF).
- Limitations: High energy costs, potential for unwanted byproducts, less effective for short-chain PFAS.
PFAS Treatment System Design
Designing a treatment system is a multi-stage process that balances innovative science with pragmatic considerations. Optimizing vessel configuration, sizing, and site arrangement for treatment technologies ensures effective and compliant remediation.
Factors To Consider In Designing PFAS Treatment Systems
Whether water providers are looking for temporary or permanent solutions, the following factors should be weighed:
-
PFAS removal capabilities: Naturally, the most critical aspect of any treatment technology is how effectively it removes the specific PFAS compounds in the water source. Technologies such as those described above have proven capabilities.
-
Water quality: The existing water quality, including other contaminants, organic matter, and water chemistry parameters, can impact the performance and effectiveness of PFAS removal technologies. These issues need to be carefully evaluated during the design process.
-
Process integration: The treatment system must integrate well with the utility's existing facilities, operations, and infrastructure. This includes evaluating available space, power requirements, waste disposal, and compatibility with other treatment processes.
-
Operational factors: The design must account for the long-term operation and maintenance requirements of the PFAS treatment system, such as the need for resin regeneration, filter replacements, and waste disposal.
-
Regulatory compliance: The treatment system must meet any applicable regulatory requirements for PFAS levels in drinking water and other water quality standards.
-
Cost: Designers must also consider the capital and operating costs of the PFAS treatment system, including expenses for equipment, construction, energy, consumables, and waste disposal.
Large-Scale Water Treatment Plants
Large-scale treatment systems should consider all the above plus the following essential criteria:
-
Treatment objectives and removal targets: Clearly define the target PFAS compounds and the desired removal efficiencies to meet regulatory requirements or utility goals. Understand the specific PFAS properties (e.g., chain length, functional groups) and how they impact removal by different technologies.
-
Treatment technology selection: Assess the effectiveness, reliability, and cost-effectiveness of varying PFAS removal technologies like activated carbon, ion exchange, and high-pressure membranes. Consider integrating multiple treatment processes for enhanced PFAS removal. Conduct bench- and pilot-scale testing to validate the performance of selected technologies under site-specific conditions.
-
Operational factors: Determine the empty bed contact time (EBCT) requirements for activated carbon systems to achieve the desired PFAS removal. Evaluate the operational feasibility, maintenance needs, and waste management implications of the selected PFAS treatment technologies.
-
Flexibility and expandability: Incorporate design features that allow for future modifications or expansion to accommodate changes in water quality, regulations, or demand.
-
Reliability and redundancy: Ensure the treatment plant has sufficient reliability, backup systems, and redundancy to maintain consistent water supply and quality in the face of PFAS contamination.
Case Studies Showcasing Effective PFAS Treatment System Designs
Despite the challenges inherent in PFAS removal, success stories offer a roadmap to utilities looking to eradicate PFAS.
Horsham Water & Sewer Authority (HWSA) Case Study
The town of Horsham, outside of Philadelphia, experienced significant PFAS contamination due to its proximity to multiple polluters but now stands as a success story. In 2014, HWSA began to assess the problem and develop a solution. At first, HWSA planned to use granular activated carbon (GAC) adsorption for PFAS removal but later decided to pilot-test ion exchange (IX) resin as an alternative.
The pilot testing showed that the IX resin had significantly higher PFAS removal capacity than GAC, with 15-21 times higher bed volumes until the breakthrough for various PFAS compounds. As a result, HWSA decided to use IX resin for the final permanent PFAS treatment systems for their remaining wells despite the initial regulatory hurdles being more difficult for IX compared to GAC. Today, Horsham’s water stands as an example of successful PFAS removal.
Cape Fear Public Utility Authority (CFPUA) Case Study
CFPUA evaluated various treatment options, including GAC, IX, and high-pressure membranes, for PFAS removal at their Sweeney Water Treatment Plant. After considering economic and non-economic factors, CFPUA selected post-filter, deep-bed GAC contactors as the best overall PFAS treatment solution. The GAC facility was designed for a 20-minute empty bed contact time (EBCT) at the maximum 44-MGD treatment capacity, requiring about 3 million pounds of GAC. If needed, the GAC contactors were also designed for potential future conversion to IX resin. Their efforts succeeded in removing PFAS efficiently.
Integrating Multiple Technologies For Maximum PFAS Removal
Combining multiple technologies allows companies to maximize their PFAS removal by selecting complementary methods that address different contamination aspects to optimize results. Through careful integration, PFAS removal strategies can address both short and long-chain compounds while minimizing waste generation and environmental impact.
Combining Filtration With Destruction Technologies
- Membrane filtration (e.g., NF or RO) concentrates PFAS.
- AOPs break down the concentrated PFAS.
This combination offers several advantages:
- Membrane filtration effectively removes a wide range of PFAS, including short-chain compounds.
- Concentrating PFAS via filtration reduces the volume of water requiring treatment by AOPs, improving efficiency and reducing costs.
- AOPs can then destroy the concentrated PFAS, converting them into less harmful byproducts.
Integrating Adsorption And Oxidation
- Activated carbon or ion exchange removes and concentrates PFAS.
- Electrochemical oxidation destroys the captured PFAS.
This approach benefits from:
- The high removal efficiency of adsorption methods for long-chain PFAS.
- Electrochemical oxidation's ability to mineralize PFAS into fluoride ions effectively eliminates the contaminants.
Best Practices For Combining Technologies
When integrating technologies, companies should consider the following:
- Specific PFAS compounds present in their wastewater
- Required treatment capacity
- Energy consumption and operational costs
- Potential for byproduct formation
- Regulatory compliance requirements
Operation And Maintenance Of PFAS Removal Systems
Operation and maintenance considerations must ensure proper system monitoring, implement rigorous maintenance protocols, and provide comprehensive training for water treatment personnel. Careful attention to these factors is essential for the long-term effectiveness of PFAS removal systems.
Operation And Monitoring
Water treatment units require regular monitoring and maintenance to ensure they continue working effectively over time. The different purification methods and materials require varying degrees of maintenance, but in every instance, improper operation or lack of maintenance can cause the systems to lose their PFAS removal effectiveness. Utilities need to closely monitor the PFAS levels in the treated water and make adjustments as needed.
Maintenance Protocols For Long-term Effectiveness
PFAS removal systems like activated carbon filters and RO membranes must be serviced and replaced regularly to maintain their performance. This includes replacing filter media, membranes, and other components as they become saturated or fouled over time. Developing and following rigorous maintenance protocols is critical.
Personnel Education And Training
Proper operation and maintenance of PFAS removal systems requires specialized knowledge and skills. Water utilities need to provide thorough training for their treatment plant staff on the technology, monitoring procedures, maintenance requirements, and troubleshooting. Ongoing education is vital as regulations and best practices evolve.
Sustainability And Safety Considerations For Managing PFAS-Contaminated Media
Strategizing for sustainability is key to managing PFAS-contaminated media efficiently. For instance, in-situ remediation approaches have proven to be more sustainable than traditional pump-and-treat methods. These in-situ techniques, such as using colloidal activated carbon barriers, have demonstrated significantly lower carbon footprints and reduced raw material, energy, and waste production compared to ex-situ treatments.
Safety considerations must also be factored into handling PFAS-contaminated media. The EPA's guidelines stress the importance of preventing PFAS releases into the environment during treatment and disposal processes. Proper emission controls are crucial for thermal destruction methods to prevent the formation and release of harmful byproducts. Landfilling PFAS waste requires robust containment systems to minimize leaching into groundwater and release through landfill gas. Underground injection, while effective for isolation, necessitates careful site selection and engineering controls to ensure long-term containment.
Because certain PFAS are designated as hazardous substances under CERCLA, companies must be vigilant in their reporting and cleanup practices to avoid significant fines and penalties.
PFAS Destruction Technologies
Once PFAS are removed, they must be carefully and safely eradicated so they do not continue to harm people and the environment. Thermal and chemical methods break down PFAS compounds into harmless byproducts, but the advantages and disadvantages of each method should be carefully considered.
Thermal And Chemical PFAS Destruction Methods
A range of thermal and chemical technologies are being developed and deployed to destroy PFAS in water, each with its own advantages, disadvantages, and optimal applications.
Thermal Degradation/Incineration
Applying high heat (980°C to 1200°C) can destroy PFAS through thermal oxidation and mineralization, breaking the PFAS compounds into carbon dioxide, water, hydrogen fluoride, and other byproducts. However, this complex process requires high temperatures and long residence times to achieve satisfactory PFAS destruction. To expedite PFAS destruction, the EPA has created a database to help water utilities understand and implement thermal processes.
Chemical Destruction Technologies
New and emerging technologies offer alternatives to thermal degradation, including:
-
Supercritical water oxidation (SCWO): High temperatures (374°C) and pressure oxidize organic compounds like PFAS. The SCWO process can effectively destroy PFAS, though it struggles with high salt concentrations and requires pretreatment.
-
Hydrothermal alkaline treatment (HALT): Similar to SCWO, this method uses high temperature and pressure along with a sodium hydroxide catalyst to destroy PFAS.
-
Non-thermal plasma (NTP): An electrical discharge generates reactive species that can degrade PFAS. NTP has shown promising PFAS destruction capabilities, though water chemistry factors like pH and organic matter can impact it.
-
Chemical oxidation: Advanced oxidation processes like ozonation, Fenton's reagent, and persulfate oxidation can chemically break down PFAS compounds. The effectiveness depends on the specific PFAS compounds and water quality.
-
Electrochemical oxidation (EO): Oxidizes and degrades PFAS compounds through direct and indirect electrochemical reactions. EO has demonstrated >99% removal of long-chain PFAS, though short-chain PFAS are more challenging and can even increase in concentration due to precursor conversion.
-
Photocatalytic defluorination: Photocatalysis uses light-activated catalysts like titanium dioxide to generate reactive species that can degrade PFAS. This method has relatively low energy requirements, but its PFAS destruction rates have been significantly lower than those of other technologies like EO.
Environmental Considerations For PFAS Destruction
Destroying PFAS responsibly includes protecting the environment from further contamination. Complete PFAS destruction, preventing the release of hazardous byproducts, managing PFAS-contaminated waste streams, and addressing the broader environmental justice implications are key factors when planning a destruction strategy. The following issues should be addressed:
-
Incomplete destruction and byproduct formation: Thermal treatment/incineration may not entirely destroy PFAS and can generate problematic byproducts like fluorinated organic compounds, HF gas, and contaminated ash/residues. Incomplete destruction can lead to the release of smaller PFAS molecules or other hazardous byproducts that can reenter the environment.
-
Air and soil pollution risks: Incineration and other thermal processes can release PFAS and other pollutants into the air, potentially contaminating nearby soil and water. Higher PFAS concentrations have been found in air, soil, and surface water near PFAS incineration facilities.
-
Waste management challenges: PFAS-contaminated waste streams, such as ash, sludge, and filters from treatment processes, must be handled appropriately to prevent further environmental release. Landfilling or deep-well injection of PFAS waste can lead to leaching and groundwater contamination.
-
Energy and resource intensity: Many PFAS destruction technologies, like supercritical water oxidation, are highly energy- and resource-intensive. The entire lifecycle environmental impacts of these processes need to be carefully evaluated.
-
Environmental justice considerations: The siting and operation of PFAS destruction facilities can disproportionately impact disadvantaged communities. Proper mitigation of environmental and health risks to these communities is crucial.
PFAS Destruction Case Study
A partnership in West Michigan has created the U.S.'s first high-volume, closed-loop PFAS destruction system. The system uses a combination of technologies, including supercritical water oxidation (SCWO), to effectively destroy PFAS on a large scale. After destruction, the treated water is cooled and discharged to the local wastewater treatment plant, meeting regulatory standards.
Engaging With The Community About PFAS
Given the immense scale of PFAS removal nationwide, local water utilities need collaboration and cooperation from their communities to succeed. With the right strategies, utilities can foster trust, manage risks, and create a collaborative relationship with their communities throughout the remediation process.
Strategies For Utilities To Inform And Involve Their Communities
First, utilities should employ effective communication strategies to inform the public about their PFAS removal efforts. Proactive messaging that engages employees, local officials, and health departments ensures consistency. Educational materials that cover the water system, the specific PFAS contaminant, existing solutions, and recommended information sources should be readily available. Utilities should also leverage multiple channels to reach the public, including dedicated web pages, social media, town halls, and local news outlets.
Utilities should develop practical steps that residents can take to reduce their PFAS exposure and provide clear, timely information about PFAS testing results, potential risks, and remediation efforts using plain language. Finally, when responding to customer inquiries about PFAS, utility staff must show respect, empathy, and transparency.
Collaborating With Community Stakeholders
In addition to developing comprehensive communication strategies, utilities should involve community stakeholders to address concerns. Engaging with local leaders, environmental groups, schools, and residents can help utilities understand the community's needs and priorities around PFAS remediation. Collaborative efforts can also help secure funding for PFAS treatment infrastructure, which is critical given the cost of high compliance.
Importance Of Transparency And Trust-Building In Public Communication
Addressing public concerns and building trust is essential for successful PFAS removal efforts. Utilities should be open and honest about the challenges and limitations of PFAS treatment technologies and the compliance costs that may affect individuals and communities. Providing clear, science-based information and responding to customer inquiries can help utilities establish credibility and earn the public's trust.
Future Outlook For PFAS Removal And Discharge
Anticipated Regulation Changes
The EPA continues to take significant steps to expand the regulation of PFAS discharges from various sources through new and revised New Effluent Limitation Guidelines (ELGs) and enhanced National Pollutant Discharge Elimination System (NPDES) permitting requirements. These actions are part of the agency's broader PFAS Strategic Roadmap to address PFAS contamination.
New Effluent Limitation Guidelines
The EPA has determined that revisions to the effluent limitations guidelines and pretreatment standards for the Landfills Category must address PFAS found in landfill leachate. The EPA plans to propose new ELGs for PFAS manufacturing facilities in the Spring of 2024 and metal finishers by the end of 2024. The EPA also announced plans to develop ELGs and pretreatment standards for landfills, textile mills, and concentrated animal feeding operations (CAFOs) to address PFAS discharges.
Expanded NPDES Permitting
In February 2024, the EPA released draft guidance to help state permitting authorities address PFAS discharges through the National Pollutant Discharge Elimination System (NPDES) permitting program. This will engender comprehensive monitoring and regulation of PFAS releases to waterways from combined sewer systems.
Emerging Technologies
Emerging technologies represent significant advancements in PFAS remediation, offering more efficient, cost-effective, and environmentally friendly alternatives to traditional methods, such as:
Hydrothermal alkali treatment (HALT) and supercritical water oxidation (SCWO) can effectively break down PFAS compounds in liquid and solid waste streams. HALT uses high temperatures and pressures to rapidly degrade over 100 PFAS compounds in contaminated water and soil, while SCWO applies supercritical water conditions to destroy PFAS.
With electrochemical oxidation (EO) technology, electrodes generate oxidizing agents that break down PFAS molecules. Mechanochemical degradation (MCD) uses high-energy ball milling to physically and chemically degrade PFAS compounds in solid materials such as soil without high temperatures or pressures.
A novel plant-based technology called RAPIMER (Renewable Artificial Plant for In-Situ Microbial Environmental Remediation) combines adsorption and bioremediation. A lignocellulose framework adsorbs PFAS and provides nutrients for fungi that degrade the contaminants. RAPIMER may be a sustainable and efficient solution with lower environmental impacts compared to other commercial sorbent techniques.
Hydrodynamic cavitation uses bubble formation and collapse to degrade PFAS. Researchers at Oxford Brookes University have developed a hydrodynamic reactor that achieved nearly 36% degradation of 11 common PFAS variants in just 30 minutes without additional chemicals, showing promise for wastewater treatment applications.
Clay-based PFAS removal also shows promise as an eco-friendly and cost-effective treatment option. Natural clay minerals like phyllosilicates and oxidic clays have shown promise for adsorbing and removing PFAS from contaminated water and wastewater. Modified clay minerals, such as those treated with surfactants or amines or combined with polymers and carbon materials (e.g., biochar), have demonstrated even higher PFAS removal capacities. Mechanisms like hydrophobic interactions, electrostatic attraction, and ligand exchange drive the adsorption of PFAS onto clay-based adsorbents.
Researchers have used the partition coefficient to compare the PFAS adsorption capacity of different clay-based adsorbents. Factors like pH, temperature, competing ions, and natural organic matter can influence the PFAS removal efficiency of clay-based systems.
Challenges include improving the regeneration and reuse of clay adsorbents and enhancing PFAS removal efficiency, especially for shorter-chain PFAS compounds. Future research will focus on developing clay-carbon composite materials, like clay-biochar, which may offer improved PFAS adsorption performance.
Final Thoughts
In short, PFAS removal is a complex and expensive undertaking that nonetheless is necessary to protect public health and the environment. As PFAS removal and destruction science evolves, it's crucial for utilities to stay informed on current trends and emerging technology. Water utilities should also follow best practices when creating strategies to meet the EPA's evolving rules and guidelines for PFAS removal and destruction and hold polluters accountable for cleanup costs.
Emerging methodologies are developing and implementing technologies that effectively destroy PFAS while minimizing environmental impact and ensuring public safety. This ongoing challenge requires a balance between technological innovation, regulatory compliance, and environmental stewardship.
Finally, utilities should partner with community leaders to address health and safety concerns. Working together, utilities and communities can create cleaner, safer water for all.
Frequently Asked Questions (FAQs)
1. What are the most effective technologies for removing PFAS from drinking water?
The most effective technologies for removing PFAS from drinking water are:
- Activated carbon adsorption, especially GAC
- Ion exchange resins
- High-pressure membrane filtration (like reverse osmosis)
These methods can remove over 99% of PFAS in lab and real-world settings.
2. How does activated carbon compare to other PFAS removal methods?
Activated carbon, particularly GAC, is highly effective and the most studied method for PFAS removal. It works well for long-chain PFAS but is less effective for short-chain compounds. While activated carbon averages about 73% PFAS removal, its performance can be inconsistent. In comparison, reverse osmosis and dual-stage filters consistently achieve over 94% PFAS removal.
3. What are the challenges of using high-pressure membranes for PFAS treatment?
High-pressure membranes like R) are very effective at removing PFAS, including short-chain compounds. However, RO systems face challenges:
- High capital costs
- High energy demand
- Susceptibility to membrane fouling
- It may require pretreatment or antiscalant use
- Produce a concentrated waste stream that requires disposal
4. Can point-of-use technologies effectively remove all types of PFAS?
Point-of-use technologies vary in their effectiveness for different PFAS types:
- RO and dual-stage filters show near-complete removal for all evaluated PFAS.
- Activated carbon-based filters are more effective at removing long-chain PFAS (60-70% removal) than short-chain PFAS (about 40% removal).
- Under-sink activated carbon block filters showed mixed results, removing most PFSAs and PFEAs but only about half of PFCAs.
5. What are the latest advancements in PFAS wastewater treatment?
Recent advancements in PFAS wastewater treatment include:
- A hydrodynamic reactor using bubble cavitation, achieving 36% degradation of 11 common PFAS variants in 30 minutes without additional chemicals.
- Pretreatment processes that form hydrophobic complexes with PFAS molecules, enhancing GAC performance for both short-chain and long-chain PFAS.
- Improved activated carbon adsorption techniques, including the use of lignin-based activated carbon for better removal of certain PFAS compounds.
- Development of more efficient ion exchange resins and regeneration methods.
References:
- 12 Treatment Technologies – PFAS — Per- and Polyfluoroalkyl Substances (itrcweb.org)
- 2.2 Chemistry, Terminology, and Acronyms – PFAS — Per- and Polyfluoroalkyl Substances (itrcweb.org)
- 2024 Promises To Be a Critical Year for New PFAS Regulations | BakerHostetler (bakerlaw.com)
- 4 things to know about regulating ‘forever chemicals’ in drinking water | PBS NewsHour
- A Review of PFAS Destruction Technologies - PMC (nih.gov)
- A Review on Removal and Destruction of Per- and Polyfluoroalkyl Substances (PFAS) by Novel Membranes - PMC (nih.gov)
- Advanced oxidation processes for the degradation of PFAS. | Download Scientific Diagram (researchgate.net)
- America’s first high-volume ‘PFAS Annihilator’ is up and running in West Michigan | WOODTV.com
- AWWA Source Water Protection
- Biden administration sets first national standard to limit ‘forever chemicals’ in drinking water | CNN
- Biden-Harris Administration Finalizes First-Ever National Drinking Water Standard to Protect 100M People from PFAS Pollution | US EPA
- Coalition Report Correcting PFAS Myths.pdf (awwa.org)
- Effects of EPA Designation on CERCLA PFAS Chemicals (natlawreview.com)
- Electrochemical remediation of perfluoroalkyl substances from water (nsf.gov)
- EPA PFAS Drinking Water Laboratory Methods | US EPA
- EPA Region 2 PFAS Community Engagement Session Summary
- EPA’s Per- and Polyfluoroalkyl Substances (PFAS) Action Plan
- FACT SHEET: Biden-Harris Administration Takes Critical Action to Protect Communities from PFAS Pollution in Drinking Water | The White House
- Figure1-classification-of-per-and-polyfluoroalkyl-substances -PFASs.pdf (oecd.org)
- Identifying and Characterizing PFAS Compounds | Technology Networks
- Implications of PFAS definitions using fluorinated pharmaceuticals - PMC (nih.gov)
- Key EPA Actions to Address PFAS | US EPA
- Memorandum for Interim Guidance on Destruction or Disposal of Materials Containing PFAS in the US (osd.mil)
- Nanomaterials | Free Full-Text | Nanomaterial-Based Advanced Oxidation/Reduction Processes for the Degradation of PFAS (mdpi.com)
- National Institute of Environmental Health Sciences: Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS) (nih.gov)
- Our Current Understanding of the Human Health and Environmental Risks of PFAS | US EPA
- Per- and Polyfluoroalkyl Substance Toxicity and Human Health Review: Current State of Knowledge and Strategies for Informing Future Research - PMC (nih.gov)
- Per- and Polyfluoroalkyl Substances (PFAS) - Treatment.pdf (awwa.org)
- Per- and Polyfluoroalkyl Substances (PFAS) | US EPA
- Per- and Polyfluoroalkyl Substances (PFAS) in Drinking Water | American Association for the Advancement of Science (AAAS)
- Per-andPolyfluoroalkylSubstances(PFAS)-Treatment.pdf (awwa.org)
- Perfluoroalkyl substances (PFAS) adsorption in drinking water by granular activated carbon: Influence of activated carbon and PFAS characteristics | Request PDF (researchgate.net)
- PFAS | American Water Works Association (awwa.org)
- PFAS Analytical Methods Development and Sampling Research | US EPA
- PFAS and Health (state.mn.us)
- PFAS and Home Treatment of Water - MN Dept. of Health (state.mn.us)
- PFAS Are Still Forever (and Not Your Best Friend) (nrdc.org)
- PFAS in Drinking Water (illinois.gov)
- PFAS removal by ion exchange resins: A review - PubMed (nih.gov)
- PFAS Strategic Roadmap: EPA's Commitments to Action 2021-2024 | US EPA
- PFAS Treatment in Drinking Water and Wastewater - State of the Science (epa.gov)
- pfas_drinking_water_treatment_technology_options_fact_sheet_04182019.pdf (epa.gov)
- PFOA and PFOS, Maine Department of Environmental Protection
- Prevalence and Source Tracing of PFAS in Shallow Groundwater Used for Drinking Water in Wisconsin, USA - PubMed (nih.gov)
- Reducing PFAS in Drinking Water with Treatment Technologies | US EPA
- Research on Per- and Polyfluoroalkyl Substances (PFAS) | US EPA Research on Per- and Polyfluoroalkyl Substances (PFAS) | US EPA
- Revisiting the "forever chemicals," PFOA and PFOS exposure in drinking water | npj Clean Water (nature.com)
- Revisiting the "forever chemicals," PFOA and PFOS exposure in drinking water | npj Clean Water (nature.com)
- Sorptive removal of short-chain perfluoroalkyl substances (PFAS) during drinking water treatment using activated carbon and anion exchanger | Environmental Sciences Europe | Full Text (springeropen.com)
- Sustainability | Free Full-Text | A Critical Review on PFAS Removal from Water: Removal Mechanism and Future Challenges (mdpi.com)
- Talking to Customers and Communities About PFAS (waterrf.org)
- Tap water study detects PFAS ‘forever chemicals’ across the US | U.S. Geological Survey (usgs.gov)
- The race to destroy PFAS, the forever chemicals| MIT Technology Review
- Water | Free Full-Text | An Overview of Per- and Polyfluoroalkyl Substances (PFAS) in the Environment: Source, Fate, Risk and Regulations (mdpi.com)
EXPERT INSIGHTS ON PFAS TREATMENT
-
Opinion: Why PFAS Policymakers Should Read Past The Abstract When it comes to drinking water, sound public policy requires sound scientific research. Publication in a prestigious, peer-reviewed journal helps establish legitimacy for scientific claims in public discourse. But science is a social process, scientific standards of evidence vary across disciplines, and peer review does not guarantee validity. For readers who stop at the abstract, these distinctions can be easy to miss.
-
PFAS Are Turning Up In The Great Lakes, Putting Water Supplies At Risk — Here's How They Get There No matter where you live in the U.S., you have likely seen headlines about PFAS being detected in everything from drinking water to fish to milk to human bodies. Now, PFAS are posing a threat to the Great Lakes, one of America’s most vital water resources.
-
Water In 2026: The Nexus Of Policy, Technology, And Resilience As water systems become more circular and complex, understanding and managing the subsurface — the hidden half of the water cycle — is becoming a critical enabler of resilience. This article explores the key trends shaping this new reality, from tackling “forever chemicals” to the water strategies redefining heavy industry.
-
Building Your Water-Positive Data Center Roadmap: A Step-By-Step Implementation Guide The data center industry stands at a critical juncture. As facilities scale to meet exponential computing demands, water consumption has emerged as a defining operational challenge. Traditional approaches focused on water efficiency are no longer sufficient.
-
PFAS In Pregnant Women's Drinking Water Puts Their Babies At Higher Risk, Study Finds
When pregnant women drink water that comes from wells downstream of sites contaminated with PFAS, known as “forever chemicals,” the risks to their babies’ health substantially increase, a new study found. These risks include the chance of low birth weight, preterm birth, and infant mortality.
-
PFAS Settlements: Debunking The Myths And Revealing What's Really At Stake For Water Utilities Misinformation and confusion could prevent some utilities from benefitting from the aqueous film-forming foam multidistrict litigation (AFFF MDL) settlements. Here are five common myths about the AFFF MDL PFAS settlements and how public water systems can make the most of this unprecedented funding opportunity.
-
PFAS Policy In 2025: Why It's Time To Go Beyond Remediation The most common techniques for disposing of PFAS may no longer be good enough.
-
The Uneven Fight Against PFAS In Rural vs. Urban Water Systems Drinking water systems across America face a mounting PFAS threat with starkly different capacities to respond. Large urban utilities typically have ample resources to detect and remove these persistent chemicals from water supplies, while small rural systems operate with tight budgets, skeleton crews, and minimal technical support.
-
CERCLA And PFAS: What's The Liability For Water And Wastewater Utilities? Federal rules aim to target those liable but may miss the mark. Utilities can redirect the effort — and costs — to those truly responsible for PFAS contamination.
-
PFAS Testing Boom: U.S. Analytical Instrumentation Market Set For Rapid Expansion The U.S. PFAS analytical instrumentation market is poised for strong expansion, with a projected CAGR exceeding 20% over the next seven years, according to a new report. Key drivers in the market include rising concerns over increasing risks associated with PFAS exposure, the U.S. EPA's federal rule on drinking water, and investments to boost testing and treating PFAS in water.
PFAS TREATMENT VIDEOS
-
The Water Online Show: 10 Must‑Watch Water Trends For 2026
The latest episode of the Water Online Show's In The Flow series features hosts Travis Kennedy and Chief Editor Kevin Westerling outlining the top 10 trends shaping the water sector in 2026, based on insights from their audience, industry experts, and past guests.
-
Can We Eliminate PFAS Forever?
Dr. Rosa Yu, National PFAS Lead at Carollo Engineers, discusses the fate of PFAS as treatment technologies evolve from removal with a PFAS byproduct to complete destruction, which would eliminate the chemicals from the water cycle, thus taking "forever" out of their name.
-
PFAS BATs: What Are The Best Available Technologies For Treating "Forever Chemicals"?
Adam Feffer, National PFAS Practice Leader at Black & Veatch, lists the Best Available Technologies, or BATs, that the U.S. EPA has recognized for the removal of per- and polyfluoroalkyl substances (PFAS). According to the current rule (but subject to change), solutions to remove two specific PFAS — PFOA and PFOS — down to 4.0 parts per trillion must be implemented by 2029. Drinking water utilities will likely select among the treatment options shared in this clip.
-
Huber Unveils Bigger Dewatering Tech And Breakthrough Energy Recovery At WEFTEC 2025
At WEFTEC 2025, Huber’s Simon Randle discusses how emerging pressures in the industry—from PFAS and microplastics to shifting federal funding—are influencing everything from technology development to workforce priorities.
-
PFAS Breakthrough: How A Novel Media And A Major Partnership Are Changing The Game
At WEFTEC 2025, CycloPure and Kurita America reveal how their new partnership is accelerating PFAS removal — from innovative Dexsorb™ media and regenerative treatment facilities to turnkey deployment for communities of all sizes. With regulations tightening and public health risks unchanged, the duo explains why their combined expertise, sustainability focus, and expanding installations could redefine PFAS treatment across the U.S.















