Membrane cleaning, part 3: Selecting membrane cleaning methods and materials

Having dealt with reverse osmosis system design and configuration factors in the first part of this four-part series on membrane cleaning, and the problems of fouling in Part 2, the author now turns to chemical methods for cleaning these systems.
By Stan Lueck, president of RODI Systems Corporation
Contents
How to deal with inorganic fouling
Organic fouling and biological organisms need combined approach
More than one cleaning solution may be necessary
Experience in cleaning membranes remains limited
The number of formulations for cleaning solutions possibly is as varied as the many types of feeds that are pumped through membrane water treatment systems. Rather than examine specific formulations, let's review the types of cleaners generally used to resolve the most common fouling problems. The reader is urged to contact the membrane manufacturer for recommendations about the most appropriate cleaning solutions for the type of membrane in question. A number of proprietary solutions also available from vendors specialized in chemical cleaning practice.
How to deal with inorganic fouling
A cleaning solution commonly employed to remove inorganic substances that foul membranes is designed to use either low pH or a chelation reaction as its principal mechanism. Descriptions of these cleaning strategies follow.
Low pH. Since many inorganic fouling compounds are more soluble in acidic solutions, a cleaning solution with a low pH value may be beneficial in removing them. Although the foulant may not be completely dissolved, the solid precipitate may be partially dissolved to the extent that it can be flushed out of the membrane element feed space.
Hydrochloric acid (HCI) is most often used to lower the pH of cleaning solutions. Sulfuric acid (H2SO4) should be avoided since an increased sulfate concentration may lead to the formation of insoluble sulfate deposits. Hydrofluoric acid (HF) may be used in cases of fouling from silica polymerization. But note that HF is extremely hazardous and must be used with great caution.
Every membrane type has limits for pH, and these limits change depending upon the temperature of the cleaning solution. The user is urged to check the membrane manufacturer's specifications regarding pH limits.
Chelating agents. These chemicals have multiple ionic sites in their molecular structure. Their negative sites act as electrically charged "claws" which bind to the positive ions in the inorganic fouling matter. The chelating agents literally pull the positive ions from the solid structure of the undesirable compound, thus helping to break it down to a form that can be washed away.
Two common chelating agents are citric acid and ethylenediaminetetracetic acid (EDTA). Citric acid is used for iron removal, and EDTA can remove salts of calcium, barium and strontium. Chelating agents are usually used at neutral to high pH levels to ensure that the negative sites on the chelating molecules remain ionized until chelation takes place.
Time. Since removal of inorganic foulants usually involves at least a partial breakdown of the solid structure of the compounds, it is essential that the cleaning solution is given sufficient time to work. Put simply, this means that the process cannot be rushed. It may take days to clean a severely fouled system.
Temperature. The chemical reactions involved in the removal of inorganic fouling materials will take place much more quickly if the temperature of the cleaning solution is elevated. Make sure, however, that the membrane manufacturer's limits are not exceeded. This requires close attention, especially if low pH solutions are used. Temperature limits are usually much lower at the extremities of the pH scale. (Back to top)
Organic fouling and biological organisms need combined approach
Removal of organic fouling materials can be accomplished successfully by using a combination of high pH and detergents:
High pH. Many naturally occurring organic compounds contain carboxylic acid groups. At low to neutral pH values these groups contain a hydrogen ion, which means there is no net charge on the molecule. At high pH levels however, the hydrogen ion will dissociate and leave a negative net charge. The presence of these charges make the organic molecular much more water loving (hydrophilic).
In the case of reverse osmosis (RO) membranes, a high pH cleaning solution will alter the membrane itself. Acidic groups on the membrane molecule will dissociate at high pH and result in a more hydrophilic membrane. This may aid in membrane cleaning, depending upon the chemical nature of the fouling substance.
Sodium hydroxide (NaOH) should be used to adjust the pH of the cleaning solution. If at all possible, RO permeate or deionized water should be used for high pH cleaning. Tap water may have sufficient calcium (CA 2) and bicarbonate (HCO-3) present to cause the precipitation of calcium carbonate (CaCO3) when the pH is increased.
Detergent. Just as in the common case of home laundry, detergents can aid in membrane cleaning. The detergent molecule is very hydrophilic at one end and water hating (hydrophobic) at the other end (see figure). This means the detergent will act as a go-between and allow water molecules to dissolve organics that normally would not interact with them.
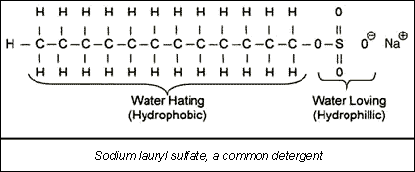
Many generic detergents can be used to clean membranes. However, care should be taken to avoid the use of laundry formulations, which may contain oxidizers (e.g., bleach). These may harm thin film composite (polyamide) RO membranes. Also, excessively high concentrations of the detergent in the cleaning solution may only aggravate the existing fouling problem. Concentrations of 0.1 to 5.0% by weight are sufficient.
Sanitizing agents. In some cases, biological organisms can be removed with a high pH detergent solution just as organic fouling can. Biological organisms are, of course, made up of organic compounds. However because of the defensive mechanism possessed by many organisms, some extra help may be necessary in removing them from a membrane treatment system.
Sanitizing agents are used to kill the organism and to help break up the biomass on the membrane and feed spacers in the membrane module. A number of proprietary products are available for this purpose. Weak solutions of hydrogen peroxide or sodium hypochlorite also can be used in certain cases. Caution is urged, however, since these are strong oxidizers and can harm certain membranes, such as polyamides. Always check with the membrane's manufacturer to determine which sanitizing agents are acceptable and under what conditions they may be used.
The sanitizing agent normally is used separately from the cleaning solution. For example, a high pH detergent solution may be used first. Next, a sanitizing agent can be applied to kill the microorganisms. Finally, another high pH detergent treatment will wash away the dead biomass.
Flow. High pH solutions are useful for removing many fouling materials that do not dissolve in a particular cleaning solution (e.g., colloidal inorganics, biomass). For this reason, flow is an important consideration in using high pH cleaning solutions. Sufficient flow has to be available to rinse the foulants from the membrane surface and the element feed space. Care should always be taken not to exceed the element manufacturer's specified pressure drop limits.
Temperature. As with low pH cleaners, elevated temperature speeds up the process in high pH cleaning. Always adhere to the membrane manufacturer's limits for temperature based upon the pH of the solution being used. (Back to top)
More than one cleaning solution may be necessary
It is important to note that, even though the types of fouling have been considered individually, real life situations don't necessarily occur in that fashion. In fact, most problems result from a combination of foulants such as silt or clay that are bound together on the membrane or on the element's feed spacers with organic material. Also, inorganic salts may precipitate on the surface of the membrane and be covered with a layer of microorganisms. In other words, fouling problems are usually not simple, and it is important to keep this in mind when choosing a cleaning strategy.
A common practice is to use high and low pH solutions in some combination. First the low pH solution can be fed into the system to remove inorganic scale or colloidal matter that may be soluble at low pH values. Then the system is rinsed to remove any of this solution that remains. Finally, a high pH solution takes out any remaining insoluble inorganic colloidal material, organic material, or biological organisms. The high pH step may be augmented by the addition of a sanitizing agent. This low pH/high pH cleaning process may have to be repeated several times in cases where fouling is severe.
It is important to rinse the membrane treatment system thoroughly with RO permeate when the transition is made from one type of cleaning solution to another. If rinsing is incomplete, minerals dissolved during the low pH step will re-precipitate when they are exposed to the high pH, or vice versa. (Back to top)
Experience in cleaning membranes remains limited
Chemical cleaning of membrane treatment systems is a difficult and incompletely understood subject. A contributing factor to the complexity of the situation is the complex nature of the fouling problem that initiates the cleaning in the first place. Many membrane treatment system operators are disappointed when they cannot find the "magic bullet" that will solve their cleaning problems. Unfortunately, the best cleaning solution often is found only by trial and error. On the positive side, trial and error can be minimized if there is a basic understanding of the various kinds of fouling that occur, and of the types of solutions that can be used to counteract them.
The fourth and final part of this series, to appear soon, will cover equipment that can be used to clean membrane systems that have become fouled.
Read the complete series of articles:
Part 1: Before you clean RO systems, you should understand them
Part 2: Fouling problems cause reduced RO system performance
Part 3: Selecting membrane cleaning methods and materials
About the Author: Stan Lueck is President of RODI Systems Corporation. You can contact him at 936 Highway 550, Aztec, NM 87410; Tel: 505-334-5865; Fax: 505-334-5867. (Back)
