What You Need To Know About Peracetic Acid (PAA) For Water Treatment
Chlorine compounds continue to be the go-to chemicals for disinfection in both pretreatment and post-treatment applications. But chlorine has many drawbacks, including the formation of disinfection byproducts (DBPs), many of which are known to have long-term carcinogenic properties. In addition, chlorinated water that enters the environment can be harmful to plants, insects, and animals alike.
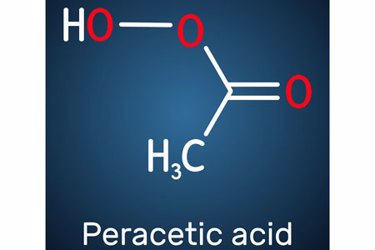
This has led to a growing interest in peracetic acid (PAA) as an alternative to chlorine for disinfection in some applications. PAA has a unique chemistry that is highly effective against bacteria and other contaminants. It does not form any known DBPs, reacts instantly with contaminants, and breaks down into acetic acid and water, making it more environmentally friendly and less prone to regulation.
An increasing number of water and wastewater treatment plants are beginning to include this chemical in their process. Before doing so, however, it is important to understand the advantages and shortcomings of PAA, as well as how to properly handle and administer it.
Get unlimited access to:
Enter your credentials below to log in. Not yet a member of Water Online? Subscribe today.
