The Differences Between UV AOP & Granular Activated Carbon For Contaminant Treatment

Providing safe drinking water is a growing challenge. While methods for the treatment of bacteria, protozoa and viruses in drinking water are well established, there are certain chemical contaminants of concern resistant to traditional water treatment methods which are being detected in drinking water, and many have the potential to impact public health.
An example is 1,4-dioxane, a stabilizing compound and a frequently-detected contaminant in groundwater aquifers due to past improper disposal practices by industry. While generally short-lived in the air, high solubility in water makes it difficult to remove 1,4-dioxane from contaminated groundwater. As a result, 1,4-dioxane is known to linger as large plumes of contamination which can migrate through groundwater aquifers and potentially be collected at extraction wells.
1,4-DIOXANE HEALTH EFFECTS & REGULATIONS
The United States Environmental Protection Agency classifies 1,4-dioxane as a probable human carcinogen with a concentration of 0.35 µg/L in drinking water representing a one in a million cancer risk level. There is no federal maximum contaminant level (MCL) in place to regulate 1,4-dioxane in drinking water, but several states, such as Michigan, have established their own guidelines and reporting limits. New York and New Hampshire are two U.S. states that are in the process of developing regulated limits on 1,4-dioxane1,2.
TREATING 1,4-DIOXANE WITH ACTIVATED CARBON FILTERS
Granular activated carbon (GAC) is made of carbon media subjected to extreme heat and oxidation which makes it very porous on the surface. These pores increase the surface area on the carbon, providing more accessible sites for contaminants to adsorb to. Many contaminants are effectively removed from water through GAC adsorption. However, 1,4-dioxane’s high solubility in water inhibits adsorption, significantly reducing the effectiveness of GAC filters. 1,4-dioxane breaks through GAC f ilters more rapidly compared to other contaminants3, resulting in more frequent replacements of the filter’s media.

TREATING 1,4-DIOXANE WITH ADVANCED OXIDATION USING UV TECHNOLOGY
Contaminant removal utilizing the ultraviolet (UV) light advanced oxidation process (UV AOP) does not physically remove contaminants from the water like GAC does. Instead, UV light – alone or in combination with an oxidant – breaks chemical bonds to destroy chemical contaminants in water. When UV light is introduced into the water, it can be absorbed directly by targeted contaminants and it can also interact with certain oxidants to generate powerful radicals which rapidly interact with and break down oxidizable contaminants, including but not limited to 1,4-dioxane. UV AOP can also simultaneously treat multiple contaminants. For example, a treatment plant targeting volatile organic compounds (VOC) like trichloroethylene (TCE) or tetrachloroethylene (PCE) can also use UV AOP for their removal, in addition to removing 1,4-dioxane.
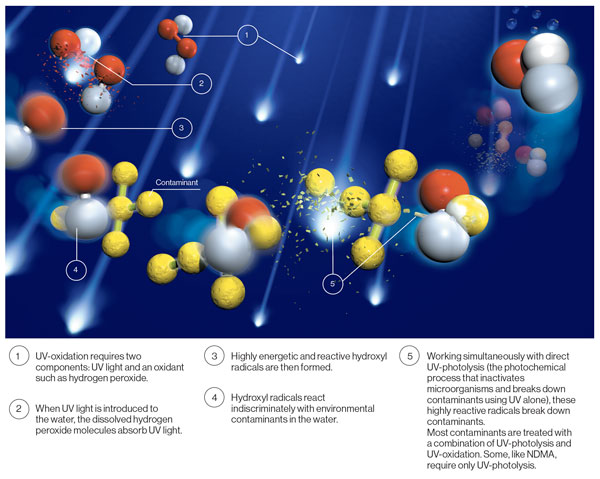
CAN UV AOP BE ADDED TO GAC FACILITIES TO TREAT RESISTANT CONTAMINANTS LIKE 1,4-DIOXANE?
Yes. A treatment plant with GAC filters designed to remove VOCs like TCE or PCE might need to consider incorporating UV AOP if 1,4-dioxane is eventually detected in GAC effluent. Since UV AOP is a chemical-breakdown process, there are no concerns for filter saturation or contaminant breakthrough. Oxidizing radicals generated by UV AOP easily react with even small molecular weight contaminants like 1,4-dioxane, and degrade them into non-harmful components.
WHAT IS THE IMPORTANCE OF OXIDANT QUENCHING IN UV AOP?
Some oxidants used in UV AOP, such as hydrogen peroxide, will not fully be converted to radicals by UV light and any unconverted oxidant will typically need to be removed from the treated water before it enters distribution. This process of removing residual oxidant is called “quenching.”
GAC is one recommended option for this quenching step to degrade residual oxidants. This is accomplished through natural catalytic activity on the surface of GAC media. GAC is also documented to react with some oxidants to produce more oxidizing radicals4 which can also facilitate quenching. As a result of these mechanisms, GAC filters used for oxidant quenching are generally more efficient, requiring reduced empty bed contact times (EBCT) compared to filters being used for direct adsorption of 1,4-dioxane or other contaminants. Further, as GAC converts to biological activated carbon (BAC) due to natural biological growth on GAC media, oxidant quenching is enhanced through biological catalytic activity5 and this extends the lifetime of the GAC media.
CAN EXISTING GAC FILTERS BE REPURPOSED FOR OXIDANT QUENCHING?
Yes. GAC facilities incorporating UV AOP are encouraged to repurpose existing GAC filters used for direct adsorption of contaminants, and instead utilize them for oxidant quenching after treatment with UV AOP. This repurposing process assigns primary treatment of chemical contaminants to the UV Advanced Oxidation system, with GAC maintaining functionality in a less intensive quenching role. Repurposing GAC can conveniently be done without changing the size or bed volume of the existing filters, and when this process is complete, repurposed GAC filters will require fewer media replacements.
CASE STUDY – LLANGOLLEN WELLFIELD, NEW CASTLE COUNTY, DELAWARE
The Llangollen Wellfield, managed by Artesian Resources, in Delaware operates at a maximum treatment capacity of 2.2 million gallons per day. Chemical contaminants including bis(2-chloroethyl) ether (BCEE) were discovered in 2000 and were initially mitigated through the installation of a GAC filter. By 2013, 1,4-dioxane was also discovered in the wellfield. Two complete GAC media change-outs for all three pairs of vessels were required each year to treat water in the wellfield at a total operating cost of $360,000 USD annually. A UV AOP system was established at the Llangollen Wellfield site as a way to treat the 1,4-dioxane as well as the BCEE with the existing GAC filters being positioned after the UV AOP system and repurposed for the quenching of residual hydrogen peroxide, which was used as an oxidant. Since the commissioning of the site in October 2014, the UV AOP system has maintained 1,4-dioxane concentrations below the desired limits and the annual cost of maintaining the GAC filters has been reduced by over $300,000 USD annually.
References:
- New Hampshire Department of Environmental Services. (2019). 1,4-Dioxane and Drinking Water (WD-DWGB-3-24) . Retrieved from https://www.des.nh.gov/ organization/commissioner/pip/ factsheets/dwgb/documents/dwgb-3-24.pdf
- New York State Department of Health. (2018). Drinking Water Quality Council Recommends Nation’s Most Protective Maximum Contaminant Levels for Three Unregulated Contaminants in Drinking Water. Retrieved from https://www.health.ny.gov/press/releases/2018/2018-12-18_drinking_water_quality_council_ recommendations.htm
- Summer, Kennedy, A.M., Knappe, D.R.U., Reinert, A.M., Fotta, M.E., Mastropole, A.J., Corwin, C.J. and Roccaro, J. (2014). Evaluation of Available Scale-Up Approaches for the Design of GAC Contactors. Water Research Foundation (Web Report # 4235). Denver, Colorado.
- Khalil, L.B., Girgis, B.S. and Tawfik, T.A. (2001). Decomposition of H2O2 on activated carbon obtained from olive stones. J. Chem. Technol. Biotechnol. 76 (11), 1132.
- Wang, F., van Halem, D., Liu, G., Lekkerkerker-Teunissen, K. and van der Hoek J.P. (2017). Effect of residual H2O2 from advanced oxidation processes on subsequent biological water treatment: a laboratory batch study. Chemosphere. 185, 637-646.
