Synthetic Adsorbents Show Promise for Removing MTBE from Drinking Water

In the first of two articles on promising process options for the removal of MTBE (methyl-tert-butyl-ether) from drinking water, synthetic adsorbents are considered and judged to be cost effective tools for the task of removing this contaminant directly from solution to meet drinking water standards.
By Paul M. Tornatore, Dr. Susan E. Powers, Dr. William J. Cooper and Eric G. Isacoff
Contents
Synthetic adsorbents effective in removing VOCs
Regeneration capability adds to life expectancy and improves economics
Where we are and what lies ahead
Due to concerns about the adverse health affects and objectionable aesthetic qualities associated with MTBE in drinking water, viable cost-effective treatment options for MTBE-contaminated groundwater are required (Cooney 1997; USEPA 1998). The polar oxygen atom in MTBE [CH3-O-C(CH3)3] causes the molecule to be much more hydrophilic than other alkane and aromatic hydrocarbons in gasoline. MTBE's high water solubility, low Henry's law constant and low organic carbon partition coefficient are indicative of its hydrophilic nature. Thus, treatment technologies that depend on the hydrophobic properties of contaminants are generally ineffective for the removal of MTBE from drinking water. For example, use of air strippers requires substantially higher air flow than for other gasoline constituents, and the adsorption efficiency for MTBE on activated carbon has been reported to be less than ten percent of that for other gasoline contaminants (Sevilla et al., 1997).
Significant research and applied development projects are underway in search of cost effective treatment alternatives for MTBE-contaminated drinking water supplies. One promising alternative under evaluation includes sorption with synthetic media. This treatment alternative has now been demonstrated on a pilot scale, addresses MTBE, tert-butyl alcohol (TBA) and other MTBE reaction intermediates, and has the capability of achieving drinking water standards at low operating costs.
Synthetic adsorbents effective in removing VOCs (top)
Synthetic carbonaceous media have been shown to be technically effective for treating VOC (volatile organic compound)-contaminated water (Hradil et al. 1991; Hand et al. 1994; Gallup et al. 1996). These media are similar to ion exchange resins but without the ionic functional groups. Preliminary work by Malley et al. (1993b) and more substantial work by Davis and Powers (2000) have shown that synthetic carbonaceous media have greater capacities for capturing MTBE than activated carbon. Unlike carbon, synthetic adsorbents have a more uniform shape, and properties controlled by manufacturing processes used in their formation (See photograph).
Courtesy of Rohm and Haas
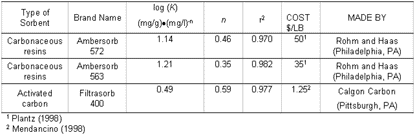
Davis and Powers (2000) performed batch equilibrium adsorption experiments to determine the effectiveness of carbonaceous media for removing MTBE from water. Two such products manufactured by Rohm and Haas Company (Ambersorb 563, Ambersorb 572) and an activated carbon (Filtrasorb 400) were considered (See table). Because Filtrasorb 400 is commonly used for removal of gasoline hydrocarbons from contaminated groundwater (Malley et al. 1993a), this was chosen as a suitable activated carbon for comparison. Competitive adsorption effects for bi-solute solutions of MTBE and m-xylene were also considered to more accurately assess the effectiveness of these sorbents, and the effects of multi-constituent contaminant streams.
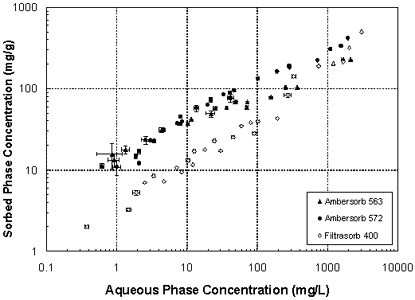
The figure shows isotherm data for MTBE adsorption to the three adsorbents considered in this study. The capacity of the two Ambersorb resins is clearly higher than Filtrasorb 400 at the lower end of the concentrations considered. Capacity differences are less at high MTBE concentrations, but an advantage to the synthetic media exists across the range of conditions evaluated, and particularly at typical MTBE concentrations in groundwater.
Common MTBE concentrations detected in groundwater are lower than many of the test cases, suggesting that Ambersorb 563, which has the highest capacity at 1 mg/l, would be the most effective sorbent. Above approximately 600 mg/l, the point at which the Ambersorb 563 and Filtrasorb 400 isotherms intersect, Filtrasorb 400's capacity is effective. A comparison of the Freundlich isotherm parameters (See table) illustrates that the sorption capacity factor (K) for the Ambersorb 563 is 5.2 times greater than that of the Filtrasorb 400. Estimated Freundlich parameters for the activated carbon compare well with values reported by other researchers (Brown et al. 1997).
Single solute isotherms alone are useful to determine if a sorbent shows promise for removal of a contaminant. Other contaminants present in actual field applications may, however, interfere with the adsorption of the primary contaminant. Because of the decrease in adsorption capacity associated with more complex systems, it is important to compare the sorbents under these conditions to determine the most effective compound. Bi-solute experiments were performed on the Ambersorb carbonaceous media and the activated carbon to determine their effectiveness in the presence of other gasoline hydrocarbons. m-Xylene (43.2 mg/l) was used as a representative BTEX compound.
MTBE isotherms for Filtrasorb 400 and the Ambersorb products with m-xylene follow the same trends as those in the figure, except the sorbed phase MTBE concentrations were less due to the presence of m-xylene. This was removed to below detection limits (0.1 mg/l) in all competitive adsorption experiments. MTBE, however, was detected over the entire concentration range studied. This implies that the media preferentially adsorb m-xylene over MTBE, an expected outcome. The Freundlich sorption capacities (K) were reduced by 22%, 11%, and 35% for the Ambersorb 563, 572, and Filtrasorb 400 sorbents, respectively, due to the preferential adsorption of the m-xylene. Despite reduction from competition by m-xylene, the Ambersorb 563 still had the highest adsorption capacity of the various media tested.
Regeneration capability adds to life expectancy and improves economics (top)
The life expectancy and ability to regenerate sorbents are important factors in the economics associated with the choice of a treatment method. The cost of each of these adsorbents is included in Table 1. While Ambersorb 563 performs better than activated carbon in the batch isotherm experiments, the cost is still 5.5 times that of activated carbon per gram MTBE adsorbed (at 1 mg/l in the water) when single use of virgin sorbents followed by disposal is compared. An alternative to disposal, however is to regenerate the carbonaceous media, since capacity and performance allow the use of small contactor vessels, and rapid regeneration using a simple regeneration process.
Regeneration allows re-use of the adsorbent through multiple treatment cycles, with significant economic advantage. Regeneration is inexpensive compared to the cost of replacement media or carbon—one of the primary advantages of sorbents resulting in their cost competitive application in water treatment (USEPA 1995). The development of onsite or centralized off-site regeneration processes for the Ambersorb media could make them more cost effective than activated carbon, and a preferred treatment alternative. Projected costs to regenerate synthetic media range from $0.15 to $0.35 per thousand gallons of water treated, much lower than carbon handling, disposal and replacement costs.
Sorption processes rely on mass transfer and collection of the contaminant, and therefore result in a waste stream requiring further treatment or disposal. Costs for waste stream treatment or disposal are not included in the cost projections.
Where we are and what lies ahead (top)
Synthetic adsorbents have demonstrated their ability to meet MTBE treatment objectives cost effectively in a variety of applications. The low operating cost of a process using synthetic adsorbents, and the ability to develop modular treatment systems relying on central regeneration facilities make the technology a promising future solution for smaller scale remediation, point-of-use water treatment, as well as for high volume applications.
Test work involving MTBE-contaminated water has shown the ability of these compounds to meet required MCLs (maximum contaminant levels) with lower energy requirements than competing oxidation technologies. The synthetic adsorbent process does not oxidize or otherwise change MTBE characteristics, avoiding reaction intermediates altogether.
Future work is recommended to demonstrate further the capabilities, refine the economics and improve the awareness of these treatment alternatives for MTBE removal.
In part 2 of this series, the authors will discuss high-energy electron injection as a method for achieving the destruction of MTBE so that drinking water standards can be met.
About the Authors:
Paul M. Tornatore, P.E., is vice president of the consulting firm Haley & Aldrich, Inc., Rochester, New York. Susan E. Powers, Ph.D, is on the staff of Clarkson University, Potsdam, New York. William J. Cooper, Ph.D, is at the University of North Carolina, Wilmington, North Carolina. Eric G. Isacoff is global technical manager for the Customer Solutions Group of the Rohm and Haas Company, Philadelphia, PA.
References
Brown, A., Devinny, J. S., and Davis, M. K. (1997). "A Review of Potential Technologies for the Treatment of Methyl tertiary Butyl Ether (MTBE) in Drinking Water." Proceedings of the 1997 American Petroleum Institute and National Groundwater Association Conference on Petroleum Hydrocarbons and Organic Chemicals in Groundwater: Prevention, Detection, and Remediation. Houston, TX, Nov. 12-14.
Cooney, C. M. (1997). "California Struggles with Presence of MTBE in Public Drinking WaterWells." Environ. Sci. Technol., 31(6), 269.
Davis, S.W., and Powers, S.E., (2000). "Evaluation of Alternative Sorbents for Removal of MTBE from Groundwater." In press, Journal of Environmental Engineering (to be published April 2000).
Gallup, D.L., Isacoff, E.G., and Smith, D.N., III (1996). "Use of Ambersorb Carbonaceous Adsorbent for Removal of BTEX Compounds from Oil-Field Produced Water." Environ. Prog., 15(3), 197-203.
Hand, D. W., Herlevich Jr., J. A., Perram, D. L., and Crittenden, J. C. (1994). "Synthetic Adsorbent versus GAC for TCE Removal." J. AWWA, 86(9), 64-72.
Hradil, J., Svec, F., Podlesnyuk, V.V., Marutovskij, R.M., Friedman, L.E., and Kliemenko, N.A. (1991). "Sorption and Desorption of Organic Compounds by Synthetic Polymeric Sorbents." Ind. Eng. Chem. Res., 30, 1926-1931.
Malley, J.P., Eliason, P.A. and Wagler, J.L. (1993a). "Point-of-Entry Treatment of Petroleum Contaminated Water Supplies." Water Env. Res., 65(2), 119-128.
Malley, J. P., Locandro, R. R. and Wagler, J. L. (1993b). "Innovative Point-of-Entry (POE) Treatment for Petroleum Contaminated Water Supply Wells." Final Report, USGS New Hampshire Water Resources Research Center, Durham, NH.
Mendancino, F. (1998). Calgon Carbon, Inc., Personal Communication, September 28, 1998.
Plantz, D.A. (1998). Rohm and Haas, Personal Communication, October 7, 1998.
Sevilla, A., Beaver, P.and Cherry, P. (1997). "Effect of MTBE on the Treatability of Petroleum Hydrocarbons in Water." Preprints of Papers, 213th National Meeting of the American Chemical Society, Division of Environmental Chemistry, 37(1), 403-405.
USEPA (1995). "Demonstration of Ambersorb 563 Adsorbent Technology." EPA-540-SR-95-516.
USEPA (1998). "Oxygenates in Water: Critical Information and Research Needs." EPA/600/R-98/048, Office of Research and Development, Washington D.C.
