Pros And Cons Of Wastewater Treatment Methods: Coagulation And Disinfection
By Nick Nicholas

Every stage in a wastewater treatment process is important to achieve the desired treatment results. However, primary treatment and tertiary are critical to the overall process. In the primary treatment process, solids are reduced to a large extent. Without this step, subsequent treatment would be less effective. In tertiary treatment, harmful microbiological matter is rendered killed or inactive so that it will not cause sickness to those organisms that encounter it.
These wastewater treatment methods are coagulation and disinfection, respectively. These processes can be accomplished in multiple ways, either by chemical or non-chemical techniques, and each have their own benefits and disadvantages.
Coagulation
Wastewater influents contain varying levels of total dissolved solids (TDS) and total suspended solids (TSS). Course screening and grit chambers will reduce the TSS but must be followed by a more refined solids removal process. Sedimentation and filtration are methods that have been used in the past, but these methods cannot remove many of the smallest particles.
Coagulation has become a popular method of reducing the TSS and, in some cases, TDS of wastewater. This process involves destabilizing the charged particles in the solution. Because of their similar electrical charges, the particles repel one another and prevent them from settling quickly. To destabilize this electrical charge, an opposite charge must be applied to the solution, enabling the colloids and other minerals to aggregate.
There are currently two well-known methods of coagulation:
Chemical coagulation is a well known method of particle coagulation. This process warrants the addition of a number of chemical additives to achieve the desired destabilized state. Alum, ferric chloride, ferric sulfate, ferrous sulfate, and lime are some of the additives used to neutralize the charged particles. Other supplements include polymers, which act as an aid for the aggregation of solids.
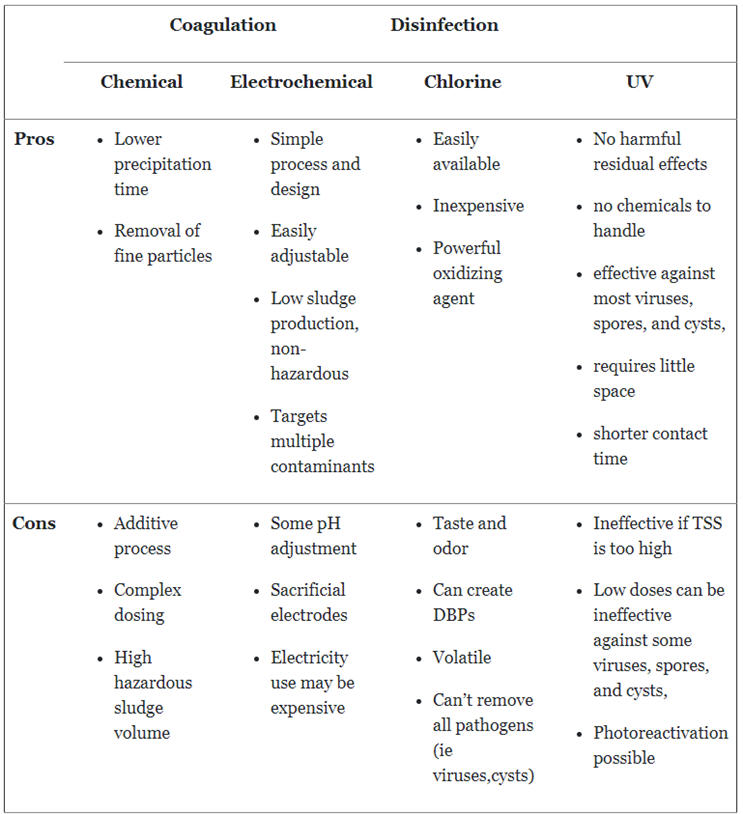
Pros
The main consideration behind the use of chemical coagulation is that it speeds up the time it would take for the solids to settle on their own. Therefore, decreasing the overall detention time of the wastewater treatment process.
Chemical coagulation can also aid the settling of finer colloidal particles and mineral contaminants. These particles typically may not settle during a sedimentation process and would pass through a subsequent filtration system.
Cons
Chemical coagulation is, at its core, an additive process. Though it can reduce the amounts of solids in a solution, it still requires the addition of chemicals to achieve this. Adding these substances can be quite complex and require extensive jar testing. The dosages need to be fairly exact in order to properly process the influent optimally. Dosage can require continuous adjustment based on the varying composition of the wastewater source.
The addition of chemicals also results in the production of a large volume of sludge that will need to be treated and disposed of following treatment. This sludge is also hazardous due to the nature of the constituents being added. The volume and toxicity of the sludge can drive up disposal costs as its not easily dewatered.
More recently, electrochemical coagulation has entered the scene in wastewater treatment in a more optimized form. After pH adjustment, if needed, this process involves the supply of specific power to a series of metallic media. The anodes and cathodes can either be the same material or different from one another. This material is optimized depending on the influent water makeup. Aluminum and iron are two such materials that can be used in this process. The electrodes release charged ions into the solution during oxidation, which leads to the destabilization of the particles in the solution.
Pros
Electrocoagulation is a straightforward process. It has few moving parts, thus it can be remotely monitored with reduced oversight and maintenance. The process can also typically be adjusted to accommodate for differing amounts of particles without much effort, if required.
The EC process is also able to target multiple contaminants using a single system and, in certain cases, with a single treatment pass. Its lack of typical chemical addition produces smaller volumes of sludge that are typically non-hazardous, easily dewatered, and less expensive to process and dispose of.
Cons
An EC system can require the addition of acids or bases for pH adjustment, so it is not completely free of additives. Also, due to the nature of the process, the electrodes are sacrificial and will corrode over time, requiring replacement. It can utilize a clean-in-place (CIP) process for plate cleaning, which would use acid in its cleaning cycle. The nature of the process also requires electrical power. While it may not require much at one time, in some places in the world the power may be more expensive, which can increase operating cost.
Disinfection
In the tertiary wastewater treatment process, the effluent may contain bacteria, viruses, mold, cysts, or other pathogens that other treatment processes cannot remove. Before the treated water can be discharged into any body of water, the microbiological contaminants need to be inactivated or killed. There are several wastewater treatment methods of disinfection available, but the two most commonly used are chlorine and ultraviolet light.
Chlorine Disinfection
Most are familiar with the use of a chlorine compound to shock-treat swimming pools. Chlorine is a toxic agent to biological organisms and kills them by oxidation. It penetrates the surface of pathogens and, once inside, begins to interact with intracellular enzymes and proteins, rendering them nonfunctional. The microorganism will either die or fail to reproduce.
Pros
Chlorine is relatively inexpensive and readily available. In addition, because it is such a powerful oxidizing agent, it can be quite effective at rendering large quantities of harmful microorganisms inert with suitable reaction time.
Cons
Chlorine is quite volatile, and can result in disinfection byproducts (DBPs) that can be harmful to humans, animals, and aquatic life. It requires careful handling to be shipped, stored, and used safely. Viruses, Giardia lamblia, and Cryptosporidium are unaffected by chlorine disinfection treatment.
UV Disinfection
Ultraviolet light disinfection systems are prevalent in many applications in recent times for their non-chemical disinfection capabilities. At particular wavelengths, UV light can disrupt a pathogen’s DNA by breaking its molecular bonds. Normal cellular function becomes impossible in this state, leaving microbiological organisms, cysts, and viruses virtually inert.
Pros
UV disinfection is an entirely physical process so there are no hazardous chemicals to handle. There is no harmful residual byproducts that could be generated in the treated water. It is highly effective against most viruses, bacteria, spores, and cysts, and requires shorter contact time than other tertiary wastewater treatment methods. In addition, it has a compact footprint for its disinfection capability.
Cons
Due to the use of light to decontaminate a solution, high concentrations of total suspended solids (TSS) can render it ineffective. This is a non-issue if the preceding treatment process is effective at removing TSS. Low doses of UV light can be ineffective against some viruses, spores, and cysts, so they would require longer contact times or higher-intensity exposure. There is also the potential for photoreactivation to occur in the microorganisms, whereby the organisms repair themselves following treatment if the UV dose is not powerful enough.
Summary Table of Pros and Cons