Nanofiltration: The Up-And-Coming Membrane Process

By David Paulson, principal partner, Water Think Tank and Prime Membrane Partners, LLC
The forgotten child of the membrane family has plenty of capabilities and potential. Learn the factors and applications that are increasing the popularity of nanofiltration.
Nanofiltration, a membrane-mediated process, is enjoying a resurgence of attention throughout the marketplace, including the potable, water reuse, and industrial process sectors. But misconceptions frequently appear about where it came from, how long it has been around, and even what the definition of nanofiltration is. A better understanding of how nanofiltration relates to its sibling processes of reverse osmosis and ultrafiltration, its history, and the potential that its current successful applications imply will make it even more useful.
The pressure-driven membrane processes are divided into four classes: reverse osmosis (RO), nanofiltration (NF), ultrafiltration (UF), and microfiltration (MF). Probably because its separation characteristics bridge the salt-rejecting and salt-passing classes, nanofiltration seems to have the most confusion in the literature and among application engineers and is inconsistently defined even in written standards. Understanding how NF was developed helps understand what it is.
Brief History Of Membrane Filtration
The modern microfiltration membrane was conceived in the early 1900s, but was consistently manufactured as the artificial, polymeric membrane we know today following World War II and has become increasingly essential in medicine, pharmaceutical production, and microbiology. RO was the next class of membranes developed and was conceived in the 1950s, developed in the ’60s, and commercialized in the early ’70s. Its target use was making drinking water from brackish water and seawater. Soon after, UF was developed and commercialized, and UF fit nicely between the salt-rejecting RO and salt-passing, particle-retaining MF. Both RO and UF needed to run in the crossflow mode to be economically viable, which created a processing liability in some cases, but the technology was a major advance.
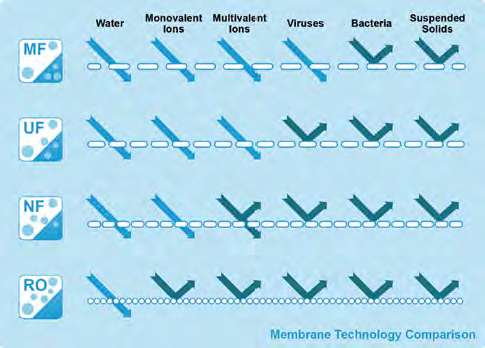
The filtration spectrum (credit: Koch Membrane Systems)
Historically, it has been understood that UF class membranes allow complete passage of ionic species, but retain uncharged solutes above 5,000 or 10,000 molecular weight through simple sieving. It was also accepted that RO membranes remove high levels of ionic species using a more complex mechanism. So, both classes of membranes removed uncharged solutes, with the “tighter” RO removing down to below 150 molecular weight (MW) while UF membranes could reliably remove solutes down to 10,000 MW for the tightest versions, and “looser” (larger pore) versions would pass such molecules but remove “macromolecular” solutes and colloids (as well as virus and bacteria).
Need For Nanofiltration
There was a tight membrane class (RO) that would remove (reject) essentially all salt ions and most uncharged organic solutes, and a group of looser membranes (UF) with smallest pore sizes from 10,000 up to 300,000-plus molecular weight cutoff (MWCO), and they worked well for many applications. But what about all those uses that both industry and science envisioned for separating one solute molecule from another in the common and valuable 500 to 10,000 MW range? And what if the purpose was not just to purify water? What membrane could a company use to desalt a protein or dye broth, to remove sugar from protein or plant matter, or to separate a mono- from a trisaccharide, etc.? An increasing need pushed the membrane manufacturers to modify their membranes to fill this gap.
A few pioneering membrane companies, especially those that focused on industrial and process applications, reacted by developing and testing what were first called “loose RO” and “RO/UF hybrid” membranes. Hence, a new category of membrane performance was realized.
Further, what if the ions (dissolved salts) that a municipality wanted removed for potable water were only the hardness ions, and the added pressure that the tight RO membranes required was an unnecessary cost? The earliest documented application of NF was a potable water application in Florida in the late 1970s and was probably the first commercial, intentional use of such NF membranes. The loose RO membrane chosen required less pressure because it allowed monovalent ions like sodium to pass through, yet still removed color molecules (and probably unrealized at the time, tri-halo-methane precursor molecules). This “membrane softening” application was born before the term nanofiltration was known.
In 1983, the first documented process NF membrane (as opposed to water treatment) I have found was commercialized for the purpose of desalting a small food-grade dye — an advantageous purification step in a critical manufacturing process. This membrane is documented well because, coincidentally, the patented use it was developed for became the basis of a major patent law interpretation case, which made it to the U.S. Supreme Court after 12 years in the lower courts (decided in 1997).
A New Membrane Class Is Born
In 1984, Dr. Peter Eriksson, in a marketing contribution to differentiate such new membranes, coined the term nanofiltration, which he based on the estimated size of the pores in a membrane with these types of removal characteristics. Thus, a fourth class of pressure-driven membranes was born.
This term and the fractionation process came into widespread use long before the “nano” materials storm that has recently swept the technology world. The only connection the NF membrane name has to nanomaterials is through the smallest size of uncharged solutes they tend to separate — a weak link.
The ability to separate some small solutes from others is a very important membrane characteristic. Keep in mind that there are two types of solutes separated by differing mechanisms: ions based chiefly on their valence state in water (charge) and sieving based on molecular weight if uncharged. This is a simplified definition that is generally not universally accepted. It seems that those industry groups that mainly purify water think only of the ionic separation performance of NF as important and often ignore the uncharged solute aspect in their definitions and common usage.
Yet some of the most creative and technical applications for NF involve removing one size uncharged molecule from a larger or smaller solute, to achieve an otherwise difficult separation. Such a process step is called “fractionation” and is employed in technical applications for food and beverage ingredients, fine chemicals, oil and gas (i.e., fracking), pharmaceutical, and biomedical applications. There will undoubtedly be more applications since the interest in using membranes is growing even faster now than in the 1980s. Many of these successful fractionation applications are kept silent as trade secrets or only show up in issued patents or patent applications, reducing the public’s understanding of how widely nanofiltration may be used in their everyday lives.
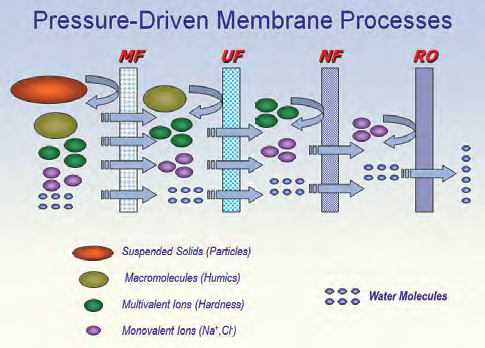
Solute rejection model (credit: Tzahi Y. Cath)
Nanofiltration Today
New and creative applications are driving the further development of NF membranes. While its siblings RO and UF continue to grow in importance for treating the world’s potable water and wastewater, NF is helping in those areas and many others as well. The need to separate related chemicals in various production streams such as pesticides, biochemicals, nutraceuticals, flavorings, and pharmaceuticals is well understood in these industries; as a result, NF is increasingly brought into play. Today several important applications for producing, refining, and recovering fine chemicals, sugars, amino acids, food, feed additives, and medicines are well established (one illogical application, making clear beer, has gone away).
The use of NF to replace RO or develop previously infeasible applications in water reclamation and reuse in both municipal applications and in the extraction industries (oil, gas, and mining) is now economically important. The reasons include reduced energy costs; reclamation of valuable minerals, elements, and chemicals; reduced volumes of toxic pollutants for disposal or further treatment; and lower-cost process streams suitable for direct disposal.
Nanofiltration Tomorrow
Although it is unlikely to reach the total area of global, installed membrane for either RO or UF, nanofiltration may well eclipse both of these classes in the number of different applications, if it has not already. The range of separations possible is not the only reason for this. Unlike the RO membrane class, comprised essentially of only two polymers, commercially available NF membranes include those of RO (cellulose acetate and polyamide-polyimides) and also more chemically resistant polymers. And more recently, ceramics companies are claiming products in the NF range. Both these material categories extend the range of NF applications significantly.
For the acid, base, and solvent stable polymeric membranes, both published lab tests and proprietary industrial applications have demonstrated that these NF class membranes can economically recover both acids and metals from acidic solutions in reuse from mining and refining streams. Closer to the economic cusp are those used for recovering acids and especially bases from food processing, industrial and commercial laundry, and other cleaning solutions, thanks to the longer life they have in these harsh environments.
For the emerging ceramic NF membranes, the same and better resistance (and therefore lifetime) can be expected — and, given the higher probable cost, is required to be competitive. To the extent that ceramic materials can withstand higher temperatures, stronger chemicals, and higher pressure, they will eclipse the polymers as the preferred membrane in high-value applications that can afford their higher cost.
Even more and potentially bigger developments are likely in the next five to 10 years. These include improved fouling resistance and customizable separation selectivity due to the incorporation of nanomaterials, for which efforts are under way in all the membrane classes (therefore nanofiltration will still not be a term properly related to the NF membranes material of construction). Just how economically feasible and how much the benefits of this technique will be are still speculative.
But one breakthrough would be of enormous benefit, and its value is assured — the development of a hollow-fiber-style NF that can be back-flushed to clean it and therefore allowed to run in direct-flow mode instead of crossflow, as with current hollow-fiber UF. This is perhaps the chief advantage ceramic NF has now, and it is probably closer to commercialization due to more favorable existing manufacturing techniques.
However, regardless of any breakthrough in materials or configuration of the NF membrane itself, nanofiltration applications are destined to multiply at a rate equal to, or probably greater than, the other membrane classes. This potential alone justifies the attention that NF has today.
About The Author
 David Paulson is principal partner in Water Think Tank and Prime Membrane Partners, LLC. Water Think Tank provides a variety of expertise in the membrane filtration and water treatment industries. Prime Membrane Partners aids companies in establishing and expanding media and component manufacturing capabilities, critical component sourcing, strategic partnerships, and optimum positioning in the marketplace.
David Paulson is principal partner in Water Think Tank and Prime Membrane Partners, LLC. Water Think Tank provides a variety of expertise in the membrane filtration and water treatment industries. Prime Membrane Partners aids companies in establishing and expanding media and component manufacturing capabilities, critical component sourcing, strategic partnerships, and optimum positioning in the marketplace.
