LC-MS/MS Analysis Of PFAS Extractables In Polyethersulfone (PES) Syringe Filters Using EPA 537.1
A key consideration for any PFAS method is to avoid contamination that can impact the accuracy of data, including those coming from sample preparation techniques such as filtration. Currently, most of the analytical methods are for “clean” matrices, such as drinking water, and often do not require filtration as a part of sample preparation. However, methods such as SW-846 Method 8327, ASTM D7968, ASTM D797 and ISO 21675 involve matrices that could have a higher degree of particulates, such as wastewater. Particulates in solution must be removed prior to LC/MS/MS, as they can be detrimental to sample analysis, column longevity and overall instrument function. These methods identify the need for filtration using membranes in a syringe filter format.
In this application note, EPA Method 537.1 was used to demonstrate that the Millex® syringe filters with PES (polyethersulfone) Millipore Express® membranes did not give any detectable levels of PFAS contamination. Figure 1 is the schematic of the experimental procedure.
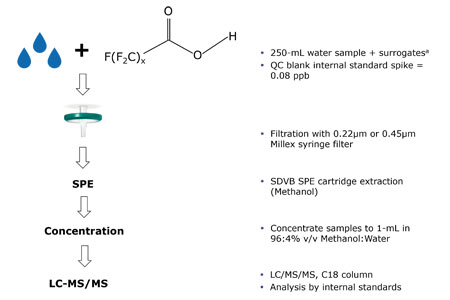
Figure 1. Schematic outline for testing Millex® syringe filters for PFAS contamination
No PFAS contaminants were detected even with the very low reporting limits (RL) of the method (Table 1). These results suggest that nonsterile Millex® syringe filters with PES membranes are reliable and appropriate to utilize in the filtration of samples for the analysis of PFAS compounds in environmental matrices that require filtration prior to further clean-up, by solid phase extraction for example, and/or LC-MS/MS analysis.
Table 1 Detection of PFAS after filtration with nonsterile Millex® filters with PES membranes using LC/MS/MS according to EPA 537.1
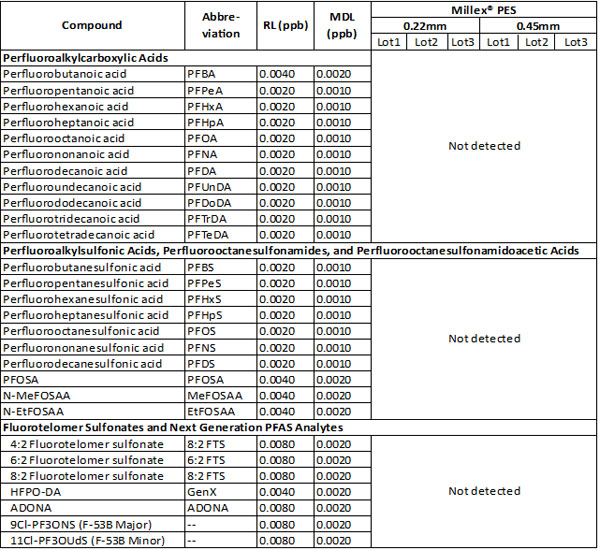
Abbreviations: RL = reporting limit (ppb); MDL = minimum detection limit (ppb).

