Industrial Water Treatment For Inorganic Contaminants: Chemical Treatment Processes

By Mark Reinsel, Apex Engineering
This article is the fourth in a series on industrial water treatment focusing on inorganic contaminants. While regulatory limits are being established, the process of identifying a cost-effective treatment process should be undertaken. Potential water treatment processes for inorganic contaminants can be grouped into three categories: physical, chemical, and biological. This article will describe five chemical treatment technologies that may be considered:
- Hydroxide precipitation
- Sulfide precipitation
- Oxidation/reduction
- Ion exchange
- Natural zeolites
Typical contaminants of concern in industrial waters include suspended metals, dissolved metals, nitrate, sulfate, and cyanide. Common metals and metalloids include arsenic, antimony, selenium, lead, copper, cadmium, and zinc.
Hydroxide Precipitation
Hydroxide precipitation is the simplest and most common method for metals removal. An alkaline agent such as lime is used to increase the pH to where metals solubilities are below the discharge limits. Either hydrated lime (calcium hydroxide or Ca(OH)2) or pebble lime/quicklime (calcium oxide or CaO) may be added. Pebble lime is less expensive than hydrated lime but requires a slaker, which hydrates the lime in an exothermic (heat-generating) reaction. Long-term operations requiring relatively high lime usage (1 to 2 tons/day or greater) generally justify installation of a slaker to utilize less-expensive pebble lime. The lime precipitation process at the Central Treatment Plant (CTP) on the Bunker Hill Superfund Site in Kellogg, ID, is shown in Figures 1 and 2.

Figure 1. Pebble lime silos in the foreground at the CTP

Figure 2. Aeration basin (mixing tank) at the CTP following lime addition
Other alkaline agents that can be used are sodium hydroxide (NaOH), commonly known as caustic soda; sodium carbonate (Na2CO3), commonly known as soda ash; and magnesium hydroxide (Mg(OH)2), commonly known as milk of magnesia. An advantage of caustic soda is that it can be purchased as a liquid so it does not require slurry preparation.
The pH target during hydroxide precipitation depends upon the contaminants of concern and their respective discharge limits. See Figure 3 for a chart of metal hydroxide solubilities versus pH. Iron precipitation requires a much lower pH than cadmium precipitation, for example.
Most metal hydroxides are amphoteric, i.e., they will go back into solution at higher pH, as shown in Figure 3. Therefore, removing several metals from solution may require a balancing act (a pH value between their lowest solubilities) or a two-step process.
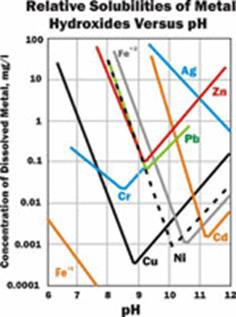
Figure 3. Metal hydroxide solubilities vs. pH
The process known as co-precipitation can also be used to increase metals removal during a hydroxide precipitation process. Co-precipitation is the “carrying down” by a precipitate of contaminants that are normally soluble under those conditions. This principle can be utilized during a water treatment process to achieve lower concentrations than might be otherwise achievable. For example, at the CTP in Idaho, the hydroxide precipitation process at pH 8.2 to 8.5 reduces cadmium concentrations from 0.2-0.5 mg/L to 0.003 to 0.006 mg/L. Figure 3 predicts that if treating cadmium alone, these effluent concentrations could only be achieved around pH 11. It is theorized that at the CTP, the high concentrations of iron and manganese precipitated (about 100 mg/L each) “carry down” the cadmium.
Sulfide Precipitation
This can be used as a “stand-alone” process if metals concentrations are low initially, or as a “polishing” step after hydroxide precipitation to achieve lower metals concentrations. Sodium sulfide (Na2S) or sodium hydrosulfide (NaHS or “Nash”) are the reagents used. A short retention time (e.g., several minutes) is required and low reagent dosage (e.g., several mg/L) are typically used at the low initial metals concentrations encountered. Figure 4 shows the lower metals concentrations that can be achieved compared to hydroxide precipitation.
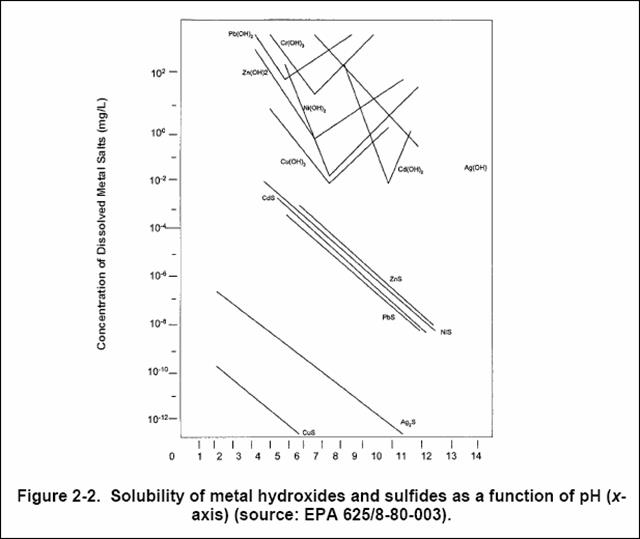
Figure 4. Solubilities of metal sulfides and hydroxides
Metal sulfides are not amphoteric so sulfide precipitation for all metals can be done in one step. Sulfide precipitation is typically done at neutral-to-alkaline pH to minimize generation of potentially toxic hydrogen sulfide gas.
Oxidation/Reduction
Oxidation/reduction reactions may be required to transform contaminants into less-soluble forms so they can be removed from solution. Oxidation is loss of electrons (e.g., oxidizing As3+ to As5+) while reduction is gain of electrons (e.g., reducing Cr6+ to Cr3+). Arsenic is typically oxidized from arsenite (AsO33- where arsenic exists in the +3 or trivalent form) to arsenate (AsO43- where arsenic exists in the +5 or pentavalent form). Oxidizing agents such as chlorine gas, sodium hypochlorite, hydrogen peroxide, ozone, or potassium permanganate are added to oxidize arsenic. Reducing agents such as sodium bisulfite or sodium metabisulfite are added to reduce contaminants such as chromium or selenium. Hexavalent chrome is reduced to its trivalent form, while selenate (Se6+) is reduced to selenite (Se4+).
Like sulfide precipitation, oxidation/reduction reactions are typically quite rapid, with retention times on the order of minutes. One cautionary note is that chemical addition will increase the total dissolved solids (TDS) concentration due to addition of ions such as sodium. This may be a concern if a TDS limit must be met.
Ion Exchange
Ion exchange (IX) is a common technology for both potable and industrial water treatment. It is a physical/chemical process that is being grouped with chemical treatment technologies for this article. A water softener, which is the most common ion exchange process, is shown in Figure 5.
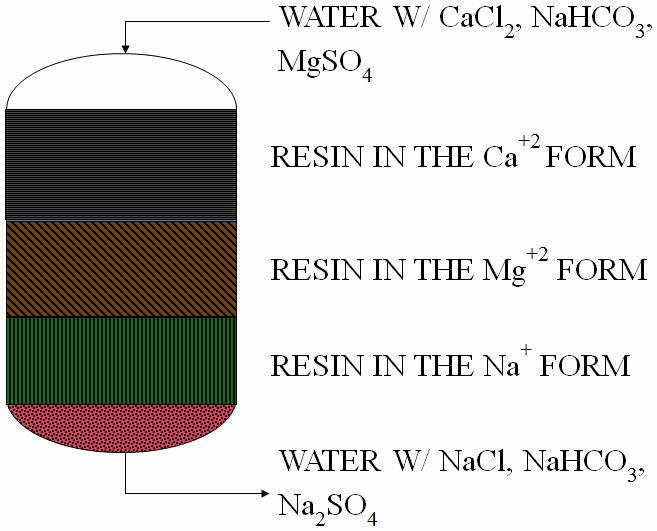
Figure 5. Ion exchange process showing resin transformation from the sodium (Na+) form to calcium and magnesium forms as water hardness is removed
Specific ion exchange resins are available for dissolved metals, arsenic, and nitrate. Sodium or hydrogen (depending on whether the resin is in the sodium or hydrogen form) are exchanged for cations (positively-charged ions such as most metals) that are removed. For anions (negatively-charged ions such as nitrate), chloride, or hydroxide ions replace the contaminants.
Several major resin manufacturers supply resin in the U.S. and internationally. Resin is relatively expensive initially (several hundred U.S. dollars per cubic foot) but can be regenerated for multiple uses. On-site regeneration of cationic resins is typically done with acid or a sodium chloride solution, while a base or sodium chloride solution is used for anionic resins.
The waste created from IX regeneration is called regenerant and it contains the contaminants removed in a more concentrated form. The regenerant volume (gallons per day) depends on the regeneration frequency, which is dictated by the contaminant concentrations, discharge limits, and resin selectivity. Either co-current or countercurrent regeneration can be used — each has its advantages. IX is a downflow process (Figure 5), so co-current regeneration will also be downflow while countercurrent will be upflow. Regenerant waste volumes are typically much lower than those for reverse osmosis (RO) or other membrane processes. Typical IX resin capacities in kilograins per cubic foot (kgr/ft3) are:
- Weak acid cation:45 to 60
- Strong acid cation:12 to 30
- Weak base anion:19 to 28
- Strong base anion I:10 to 18
- Strong base anion II:18 to 22
New IX equipment at the Buckhorn Mountain Mine near Republic, WA, is shown in Figure 6. This was a four-step process, with each of the first three vessels containing resin specific to metals, arsenic, and nitrate removal, respectively. The fourth vessel contained natural zeolites, which will be discussed next.

Figure 6. IX process at the Buckhorn Mountain Mine water treatment plant
Natural Zeolites
Natural zeolites are an inexpensive, readily available material that can be used to remove cationic contaminants such as ammonium and dissolved metals. Zeolite minerals include clinoptilolite (the material used in cat litter) and chabazite. Natural zeolites are far less expensive than IX resins, with typical costs of $0.10 to 0.40/lb.
Zeolites have the highest selectivity for thallium, i.e., they will preferentially remove thallium when brought into contact with water having multiple dissolved contaminants. The selectivity order for natural zeolites is: Tl+ > Cs+ > K+ > Ag+ > Rb+ > NH4+ > Pb2+ > Na+ = Ba2+ > Sr2+ > Ca2+ > Li+.
Natural zeolites can be purchased in a variety of size ranges, such as 8x20 or 16x50 mesh. Smaller material (higher mesh sizes) will have more available surface area and a higher adsorption capacity, but may impede flow. Chabazite is the most porous natural zeolite, with a surface area of 500 to 600 square meters per gram.
Similar to IX resin, zeolites can be regenerated with salt for repeated use. As an alternative, the material is so inexpensive that it can be disposed of when saturated with contaminants, typically as a nonhazardous waste. Valuable metals could be recovered from the regenerant or from smelting the spent zeolites. Full-scale treatment systems have achieved up to 0.4 percent thallium loading by weight at an influent thallium concentration of 0.6 mg/L, with higher loading possible at higher influent concentrations.
Chabazite is stable at pH values of 4 and above, while clinoptilolite is stable to a pH of 3. Material bulk densities are 40 to 60 pounds per cubic foot.
For more information, contact Mark Reinsel at http://apexengineering.us/.
