Industrial Wastewater Treatment Options For Inorganic Contaminants
By Mark Reinsel, Apex Engineering
With a plethora of contaminants deriving from a multitude of sources and a variety of treatment solutions to choose from, the topic of industrial wastewater can get complex fast. This overview provides clarity.
Industrial wastewater treatment for inorganics can be as simple as settling or filtration and as complex as multistage chemical precipitation or ion exchange processes. Technologies continue to evolve; the following methodology is recommended for selecting the best technology for each application, and several proven technologies have been shown to be effective for the water quality parameters most commonly regulated. Typical parameters requiring treatment in industrial wastewater include suspended solids, dissolved metals, nitrate, ammonia, arsenic, and sulfate.
This article will be a high-level examination of the treatment options available for inorganic contaminants.
The same basic steps can be followed in selecting a process for most industrial wastewater treatment applications:
- Evaluating and confirming the design criteria;
- Reviewing potential treatment technologies to address those criteria;
- Developing one or more process flow sheets;
- Estimating capital and operating costs for one or more options; and
- Performing bench and/or pilot tests.
Design criteria include average and maximum anticipated flow rates, influent concentrations, and effluent concentrations (discharge permit limits). Concentrations may be unknown early in the design process but can be estimated through modeling or by examining similar sites. It is important at this point to analyze for both total and dissolved contaminants.
Treatment Limits
A logical starting point is to examine the regulations that determine (or are interpreted to determine) an industrial facility’s discharge limits. These limits then form the basis for all of the water treatment work that follows. Effluent limits allow the environmental professional to specify treatment goals and process design criteria.
Regulatory limits come from four main programs:
- For point source discharges to surface water, National Pollutant Discharge Elimination System (NPDES) permits or the state equivalent;
- For groundwater discharges, the appropriate state program;
- Underground injection control program (U.S. EPA or state); and
- Nonpoint source controls such as total maximum daily loads (TMDLs) or best management practices (BMPs).
In the most common program, NPDES permits are generally required for discharge of pollutants from any point source into “waters of the U.S.” An NPDES permit is essentially a license or contract for discharge of specified amounts of pollutants into a water body under specified conditions. Exceeding those specified amounts or conditions may bring legal and/or financial penalties.
A point source is any discernible confined and discrete conveyance from which pollutants are or may be discharged. Examples include pipes, ditches, leachate collection systems, and publicly owned treatment works (POTWs). The term “waters of the U.S.” (or State) covers a broad range of surface waters and may include hydrologically connected groundwater. This subject has been the source of numerous court cases.
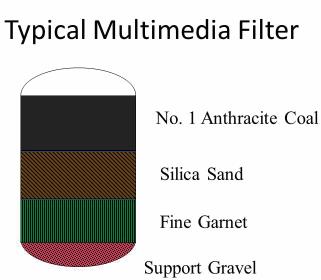
Figure 1. Multimedia filter configuration
Physical Treatment
Treatment technologies will involve physical, chemical, or biological processes. Physical processes include clarification, filtration, and membrane technologies. Except for the most rigorous membrane process (reverse osmosis), physical processes will generally not remove dissolved contaminants. Clarification uses a combination of coagulation, flocculation, and settling to remove suspended particles and typically involves sludge recycle.
Filtration methods include bag filters, cartridge filters, sand filters, and multimedia filters (Figure 1). Multimedia filters, which typically utilize anthracite coal, sand, and garnet, are probably the most common filters now in use. These filters are pressure vessels that use downflow operation to remove suspended contaminants and a periodic upflow backwash to transfer these contaminants to a waste stream.
The most common membrane technologies are microfiltration, ultrafiltration, nanofiltration, and reverse osmosis (RO). These are listed in order of decreasing pore size, increasing removal efficiency, and increasing pressure requirements. The primary disadvantage of RO is a high-volume waste stream, which often limits its applicability.
Chemical Treatment
Chemical treatment processes include hydroxide precipitation, sulfide precipitation, oxidation/reduction, ion exchange, and natural zeolites. Hydroxide precipitation typically uses lime to increase the pH. Hydrated lime or pebble lime (which requires a slaker) may be used. Other chemical alternatives include caustic soda (sodium hydroxide), soda ash (sodium carbonate), or magnesium hydroxide. For ease of addition and to avoid makeup of chemical solutions, liquid caustic soda or lime slurry is sometimes purchased.
The pH target for hydroxide precipitation depends upon the contaminants of concern. After precipitation and subsequent clarification or filtration, acid is often added to meet discharge requirements for pH. Coprecipitation, a process in which dissolved contaminants are pulled out of solution along with precipitation of high concentrations of contaminants such as iron, manganese, and sulfate, can also help to meet discharge limits.
Sulfide precipitation, which can achieve lower levels than hydroxide precipitation, is typically used as a “polishing” step to meet low metals concentrations. Sodium sulfide or sodium hydrosulfide (NaHS) is typically used. This process requires only small quantities of reagent and a short retention time. The process is typically done at neutral-to-high pH to avoid generating dangerous H2S gas.
Oxidation/reduction processes are used to transform contaminants into less soluble or more easily removed forms. For arsenic removal, oxidizing agents such as chlorine/sodium hypochlorite, hydrogen peroxide, ozone, or permanganate are commonly added. Conversely, reducing agents such as sodium bisulfite or metabisulfite may be added to remove contaminants such as chromium and selenium. Oxidation and reduction are typically rapid reactions but, since they require chemical addition, will increase the total dissolved solids (TDS) in treated water.
Specific ion exchange resins from several manufacturers are available to remove dissolved metals, arsenic, and nitrate (Figure 2). In this process, sodium or chloride ions are exchanged for the target contaminants. Resin is relatively expensive but has a long life and can be chemically regenerated (either on-site or off-site). The waste stream from ion exchange is typically much less than that generated by RO.

Figure 2. Treatment with ion exchange resins
Biological Treatment
Biological treatment processes include attached growth, suspended growth, and membrane bioreactors. Attached growth processes are most common, but membrane bioreactors are a growing application. Biological treatment can be used to remove ammonia, nitrate, selenium, sulfate, and dissolved metals.
In an attached-growth system, bacteria are attached to a media surface (Figure 3). Media can range from plastic to activated carbon to rock, with media diameters ranging from microns to centimeters. The attached bacteria (a biofilm) provide a very robust process in that it is very resilient to changes in flow, pH, and contaminant concentration. Attached-growth systems are the best choice for treating high or variable concentrations.
Suspended-growth systems are commonly used for municipal wastewater treatment but can also be used for industrial wastewater. Activated sludge is an example of suspended-growth biological treatment. Suspended growth is often used for removal of nutrients (nitrogen and phosphorus). When properly designed, these systems can be used for both nitrification (ammonia removal) and denitrification (nitrate removal). Nitrification is an aerobic process, while denitrification is anaerobic. Suspended growth is best used for relatively low contaminant concentrations.

Figure 3. Attached-growth media system
In a suspended-growth system such as activated sludge processes (also aerated lagoons and aerobic digesters), wastewater surrounds the free-floating micro-organisms, gathering into biological flocs. The settled flocs containing bacteria can be recycled for further treatment.
Suspended-growth systems typically operate poorly when encountering highly variable waste streams. Suspended-growth systems also require more energy, more equipment maintenance, and are more complex to operate because they involve more equipment than attached-growth systems.
However, attached-growth systems typically require more land, may have odor issues associated with media clogging, and may be unable to treat high wastewater flows. Consequently, urban wastewater facilities often opt for suspended-growth processes, while attached-growth processes are common in small- to medium-size operations.
Bench or pilot testing will usually determine whether the selected technologies can meet the discharge limits and may be required or “suggested” by the regulating agencies. These tests can also provide valuable information for estimating full-scale capital and operating costs. Treatment evaluations can range from jar tests performed in a day (Figure 4) to column tests lasting weeks or months.

Figure 4. Jar testing
Emerging Contaminants Of Concern
Potential emerging inorganic contaminants of concern (COCs) include:
- Methylmercury, which is one of the EPA’s National Recommended Water Quality Criteria.
- Radon. A maximum contaminant level (MCL) is being developed by the EPA.
- Cobalt, molybdenum, strontium, tellurium, and vanadium. All are included in the EPA’s Contaminant Candidate List (CCL) 3.
- Sulfate, aluminum, chloride, iron, manganese, and TDS. These all currently have secondary drinking water standards.
- Electrical conductivity and sodium adsorption ratio (SAR). These parameters are of concern in coalfield-produced water.
Emerging Technologies
Emerging technologies for inorganic contaminants include:
- Biochemical reactors for removal of sulfate, TDS, and dissolved metals.
- Enhanced solar evaporation.
- Innovative nitrate removal technologies.
- Innovative arsenic removal technologies.
Summary
Physical, chemical, or biological processes can be used to remove inorganic contaminants from industrial wastewater. Common parameters requiring treatment include suspended solids, dissolved metals, nitrate, ammonia, arsenic, sulfate, and TDS. Prior to selecting a process and designing a water treatment plant, potential treatment technologies should be investigated and bench and/or pilot tests performed.
Recommended technologies for inorganic contaminants commonly found in industrial wastewaters are:
- Suspended solids: clarification and/or filtration;
- Dissolved metals: hydroxide precipitation, sulfide precipitation, or ion exchange;
- Nitrate: attached growth biological processes (denitrification) in almost all cases;
- Ammonia: attached growth biological (nitrification), natural zeolites, or breakpoint chlorination;
- Arsenic: iron addition/filtration, iron adsorption, or ion exchange;
- Sulfate and TDS: attached growth biological or nanofiltration. Enhanced solar evaporation is an option for zero liquid discharge.
For more information, contact Mark Reinsel at http://apexengineering.us.
About The Author
 Mark Reinsel, Ph.D., PE, is president of Apex Engineering, PLLC. A process engineer with more than 30 years of experience in consulting, industry, and academia, Mark’s recent work has focused on treating mining and other industrial wastewaters through chemical, physical, and biological methods.
Mark Reinsel, Ph.D., PE, is president of Apex Engineering, PLLC. A process engineer with more than 30 years of experience in consulting, industry, and academia, Mark’s recent work has focused on treating mining and other industrial wastewaters through chemical, physical, and biological methods.
