A Practical Framework To Improve The Management Of Cyanide In Industrial Wastewaters
By Dr. Felix Zelder
Understanding your detection needs when it comes to free cyanide can help you choose the most suitable detection method.
Cyanide is an essential chemical compound for a large number of industrial processes and products that are part of our everyday lives. Unfortunately, cyanide is also one of the most lethal chemical substances known to mankind. A few hundred milligrams of this compound in solution, or ppm in air, are enough to cause severe to lethal damage in adults if ingested or inhaled. Moreover, it is particularly harmful for aquatic life and habitats.
According to the International Cyanide Management Code, the mining industry consumes about 6 percent of the annual hydrogen cyanide output (>1M metric tons) to remove gold from ore, a process that is more than 100 years old. Cyanide is also used to process foods and produce pharmaceuticals, cosmetics, and plastics. Its ability to form soluble complexes with metals such as zinc, silver, copper, and gold in water, together with its ability to aid anode corrosion and conductivity, makes cyanide an essential component of many electroplating processes.
Why Is The Management Of Cyanide Important?
Each year, the mining and electroplating industries rely on large amounts of useful, but toxic cyanide. Unfortunately, voluntary or involuntary release of this compound into surface waters does occur, posing a severe threat to animals, humans, and aquatic life. In 2000, about 100,000 cubic meters of cyanidecontaining wastewater was spilled in Baia Mare (Romania), heavily contaminating the Danube and other rivers in Eastern Europe and affecting the ecosystems, health, and economies of local communities. Tightening regulations imposed by local and regional governments to avoid similar disasters have led to increased cyanide management costs and the reevaluation of internal processes.

Mining-impaired water in Romania
For companies in the mining and electroplating industry, proper cyanide management plays a prominent role in decreasing costs, minimizing the chances of provoking environmental disasters, and avoiding bad publicity. In this context, the rapid, accurate, and safe detection and quantification of cyanide in water helps companies to control the quality of their internal processes, comply with regulations, and ensure the absence of toxic substances in their wastewaters.
Current State Of Methods For Free Cyanide Detection
Today, companies interested in the detection of free cyanide in water can choose from a wide range of techniques with variable precision, time consumption, reliability, and equipment requirements. For the exact quantification of free cyanide, techniques such as gas diffusion coupled with amperometric detection offer excellent results, but are rather time-consuming and require specialized equipment. However, colorimetric detection of free cyanide offers an attractive alternative to those interested in binary outputs: It is a semiquantitative method with great versatility in terms of speed, sample preparation, and equipment needed.
Colorimetric detection methods are based on the interaction of free cyanide with an indicator that changes color upon reaction. These methods are simple, less time-consuming, and might not require specialized equipment. They are, however, not necessarily less prone to interferences arising from other chemical species, something that, in many cases, hinders their reliability. Furthermore, several of these methods use harmful compounds, thereby putting the safety of their users at risk. The current market offerings for the colorimetric detection of free cyanide can be divided into three categories based on the technology used.
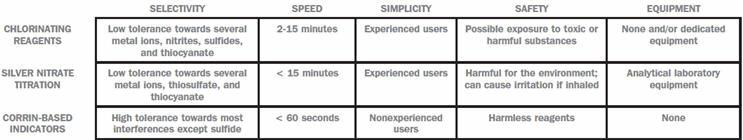
Chlorinating Reagents (König Reaction)
This traditional detection method is based on the reaction of free cyanide with chloramine T to form cyanogen chloride, which subsequently reacts with pyridine and barbituric acid to form a red-blue dye. Most commercially available free cyanide tests are based on this technology, broadly adopted by the electroplating industry. Users of this method must be aware of their possible exposure to organic solvents, harmful reagents, and highly toxic reaction intermediates.
Silver Nitrate Titration
Commonly used in both the mining and electroplating industry, this method is based on the ability of free cyanide to form complexes with silver in solution. In short, a silver nitrate solution is added to the free cyanide-containing sample, where free cyanide complexes with silver. Once all free cyanide is consumed, the excess silver ions react with an indicator (rhodamine, dithiozone, and murexide, for example) that changes color once in contact with free silver ions. However, because silver ions can also remove cyanide from other complexed species, the color might disappear after reaching the actual endpoint, thereby leading to false results. Hence, this well-established method requires experienced users and special analytical equipment, and the reagents needed can have limited bench lifetimes.
Corrin-Based Indicators
The most recent technology to detect free cyanide relies on its binding to corrin-based indicators. In this case, the indicator not only senses, but also removes cyanide from the solution. A color change of the immobilized indicator, from orange to violet, indicates cyanide’s presence in solution. This method allows for naked-eye detection of free cyanide without specialized equipment, organic solvents, and/or toxic substances. The market availability of this technology is limited, but CyanoGuard AG is currently commercializing test kits based on this method.
Why Is It Important To Evaluate Your Method Of Choice For Free Cyanide Detection?
In many cases, the complexity of your samples might hinder your detection method of choice, leading to false results, unnecessary treatments, and even involuntary disposal of cyanide into surface waters. While most methods perform well in laboratory settings, where the sample composition is known and appropriate equipment is available, their performance might vary in industrial settings. Unfortunately, this is a common situation when testing complex matrices containing known and unknown interfering chemical substances.
When using chlorinating agents, ions such as nitrites and sulfides might interfere with the indicator due to their reactivity towards chloramine T. This is a common situation in electroplating wastewaters, where these ions are usually present in high concentrations, often leading to inaccurate readings. Metal ions, such as copper, also interfere with this method and, in many commercial tests, concentrations as low as 1 mg/L are enough to alter the results.
Copper, iron, zinc, and other metal ions can also interfere with the detection of free cyanide when using silver titration methods. In gold-mining processes, where a certain minimal threshold of cyanide is needed to ensure gold extraction from ores, a trustworthy estimation of the free cyanide concentration is essential. Thiosulfate also is known to lead to overestimated concentrations of free cyanide, while the presence of sulfides leads to the appearance of precipitates and renders the detection of endpoints difficult.
Both chlorinating agents and silver nitrate titrations are sensitive to the presence of thiocyanate, a major interfering ion in most free cyanide detection methods. For chlorinating agents in particular, thiocyanate reacts with chloramine T, which is subsequently erroneously detected as cyanide. In many commercial tests, concentrations as low as 1 mg/L can already cause interferences. Moreover, thiocyanate also binds to silver ions, leading to false readings if present in concentrations higher than 10 mg/L. Therefore, determining free cyanide in samples known to contain thiocyanate can be challenging when using these methods.
A few solutions have been proposed to overcome the previously mentioned limitations, with the removal of nitrites using sulfamic acid being one of them. Yet the addition of supplementary reagents to such complicated matrices brings certain risks and can lead to the formation of new interferences. When facing similar cases, diluting the sample remains the safest solution. This option works only for samples with relatively high concentrations of free cyanide and is not recommended when detection limits as low as 0.1 mg/L must be reached.
Corrin-based indicators are a promising new alternative to bypass several limitations of the previously discussed methods. This straightforward technology is rapid, user-friendly, sensitive, and specific for free cyanide. Moreover, it works in both pure water and complex and challenging matrices. The indicator is highly tolerant to elevated concentrations of potentially interfering compounds (e.g., 200 mg/L of nitrite or thiosulfate) and is, therefore, less prone to inaccurate results. Sulfides, the only main interfering ions, can easily be removed through precipitation with ferric chloride (FeCl3) and subsequent filtration prior to free cyanide detection. This technology allows specialized, as well as nonexpert users, to determine free cyanide accurately, using nontoxic materials and minimal equipment.
A Framework To Evaluate Your Free Cyanide Detection Method Of Choice
A thorough understanding of the advantages and limitations of currently available free cyanide detection methods enables companies in the mining and electroplating industry to manage cyanide more efficiently, reduce operational costs, and safeguard local ecosystems. To facilitate the decision-making process, we propose actual and potential users to compare free cyanide detection methods in terms of their selectivity, speed, simplicity, safety, and equipment requirements. This framework can help users choose and/or reevaluate their free cyanide detection method of choice, taking into consideration their most critical needs and requirements.
References
- https://www.cyanidecode.org/cyanide-facts/use-mining
- http://www.toxipedia.org/display/toxipedia/Baia+Mare+Cyanide+Spill
- Koenig, Robert (2000). Wildlife Deaths Are a Grim Wake-Up Call in Eastern Europe. Science 287(5459):1737-1738.
- Solutions to analytical chemistry problems with clean water act methods, USEPA Office of Science and Technology (March 2007)
- ASTM D4282-15. Standard test method for determination of free cyanide in water and wastewater by microdiffusion, ASTM International, West Conshohocken, PA (2015).
- ASTM D2036-09. Standard test methods for cyanides in water, ASTM International, West Conshohocken, PA (2015).
- Zelder, F. H., Männel-Croisé, C (2009). Recent advances in the colorimetric detection of cyanide. CHIMIA International Journal for Chemistry 63 (1-2):58-62.
- Koç, E., et al. (2014). Interference of metals with the determination of free cyanide. Proceedings of 14th International Mineral Processing Symposium, Kusadasi, Turkey 1027-1033.
- Breuer, P. L., Sutcliffe, C. A., Meakin, R. L (2011). Cyanide measurement by silver nitrate titration: Comparison of rhodanine and potentiometric end-points. Hydrometallurgy 106(3):135-140.
- Alonso-González, O., et al. (2017). Free cyanide analysis by silver nitrate titration with sulfide ion as interference. Minerals Engineering 105: 19-21.
- Nagashima, S (1983). Effect of thiocyanate on the pyridine-pyrazolone method for the spectrophotometric determination of cyanide. Analytical Chemistry 55(13):2086-2089.
- EPA SW-846. Hazardous Waste Test Methods. Method 9014: Cyanide in waters and extracts using titrimetric and manual spectrophotometric procedures (2014).
- Aebli, Balz; Männel-Croisé, Christine; Zelder, Felix (2014). Controlling binding dynamics of corrin-based chemosensors for cyanide. Inorganic Chemistry, 53(5):2516-2520.
About The Author
 Dr. Felix Zelder has been a group leader at the Institute of Chemistry of the University of Zurich since 2006. He specializes in inorganic and bioanalytical chemistry and is the author of more than 11 peerreviewed papers and review articles on the colorimetric detection of cyanide in various types of samples. He is a scientific partner at CyanoGuard AG.
Dr. Felix Zelder has been a group leader at the Institute of Chemistry of the University of Zurich since 2006. He specializes in inorganic and bioanalytical chemistry and is the author of more than 11 peerreviewed papers and review articles on the colorimetric detection of cyanide in various types of samples. He is a scientific partner at CyanoGuard AG.
