A New Spin On An Old Technology Yields Surprising Results
By Daniel E. Gruenberg and Emma Flanagan
A new advanced oxidation process has been born from a quest to find a more sustainable disinfection option for high water consumption applications, revolutionizing cooling water treatment and other operations with similar objectives, as well as entirely different fields like aquaculture, wastewater treatment, and animal drinking water applications.
Oxygen is one of the most abundant elements found in nature, and living organisms need it as they oxidize nutrients to obtain energy for growth. Normal atmospheric oxygen, which makes up 21 percent of the air we breathe (O2), is known as the ground state as it’s the lowest energy level of the oxygen molecule. This atmospheric oxygen is also named by its electron configuration called triplet oxygen (see Figure 1). The O2 molecule is unique in that it has two unpaired electrons found in separate orbitals, but each with the same spin, making oxygen one of the few magnetic gases. The Pauli exclusion principle requires that no two electrons occupying the same orbit can have the same quantum state, so each electron in the same orbital must have the opposite spin.
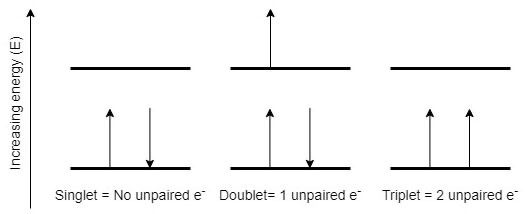
Figure 1. The three energy levels of oxygen
A flip of an electron spin in the outermost layer of the molecule will result in an excited singlet state oxygen (1O2), a potent oxidant very efficient in the inactivation of pathogens and the degradation of organic contaminants. In order for ground state triplet oxygen to become singlet oxygen, two things have to occur. Firstly, one of the 2p electrons must flip spin state, and secondly, it has to move from the Pz orbital to the Px orbital. These actions are typically induced by a photosensitizer molecule reacting with ground state oxygen (3O2). In Figure 2, ground-state oxygen is shown with orbitals Px on the left side and Pz on the right, with the same spin on the outermost level. The singlet oxygen is represented after the electron on the ground state’s right-side orbital (Pz) had changed spin direction and paired with the electron on the left side orbital (Px).
An illustration depicting the orientation of the orbitals in geometrical molecular space is shown in Figure 3. Each orbital occupies two mirror lobe shapes. Px orbital is oriented along the x-axis, Py orbital is oriented along the y-axis, whereas Pz orbital is oriented along the z-axis.
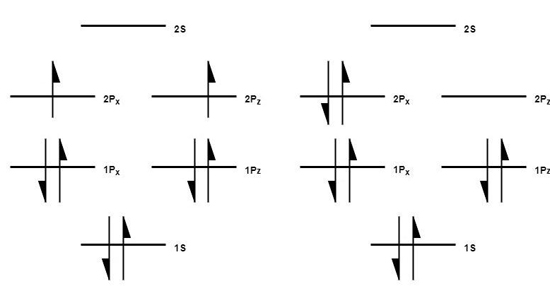
Figure 2. Orbital spins of triplet and singlet oxygen
The triplet and singlet oxygen spin layout is shown in Figures 4 and 5. The images show triplet and singlet states oxygen electrons in their 2px and 2pz, outermost orbitals.
Singlet oxygen is a powerful oxidizing agent, and it is very reactive and unstable. The half-life is less than 0.04 microseconds, but before it reverts to its triplet state, it produces other reactive molecules when it contacts water. The primary reactant is hydroxy radicals, but a complex slew of other reactive molecules is formed in water which, in turn, produce further oxidation reactions in aqueous solutions.
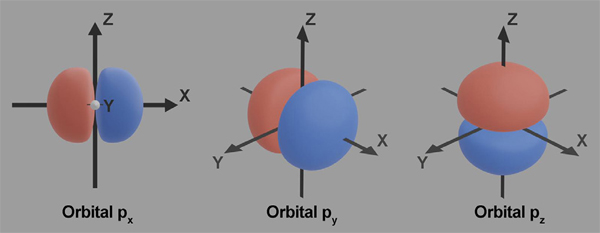
Figure 3. Orientation of the orbitals in molecular space
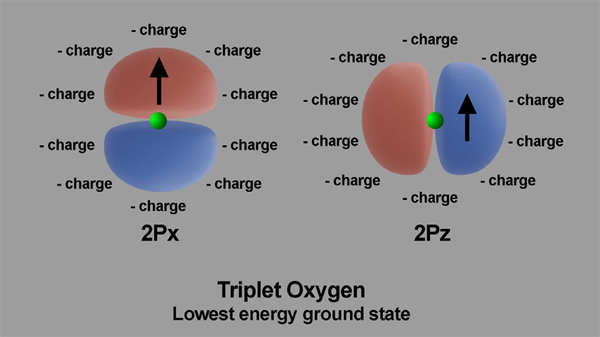
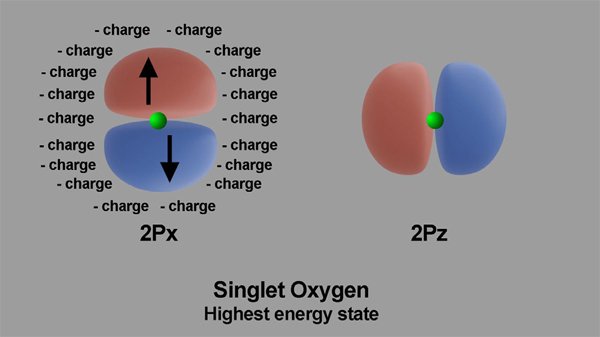
Figures 4 and 5. Arrangement of electric charges in ambient oxygen and in excited oxygen
Singlet Oxygen Is Extremely Useful
Singlet oxygen is an historically interesting molecule. It was first identified in the 1930s, although many aspects of its chemistry have emanated from the work of Christopher S. Foote, an American chemist, who conducted experiments in the 1960s where evidence was obtained supporting 1O2 generation via two independent routes, a photochemical reaction (dye-sensitized photooxidation), and a chemical reaction (NaOCl with H2O2). The biological importance of the singlet form as a reactive oxygen species (ROS) has only been discovered since the 1980s, and it plays diverse roles in photosynthesis, cytotoxicity, and oxidation of various biochemicals.
When oxygen-based oxidation is investigated, the mainstay ROS has been ozone, O3. Ozone is not as reactive as singlet oxygen and it has low solubility in water, reducing to impractical levels above 40°C. Ozone is relatively easy to manufacture in small quantities, but the feasibility of commercial production comes with drawbacks when requiring large industrial amounts. Ozone can only achieve advanced oxidation with the addition of catalysts, UV, or less effectively with the assistance of pH adjustment. While some chemists have postulated about singlet oxygen’s possible appeal, it has not yet been made commercially available. Its attractiveness stems from its reaction with water being an electrochemical reaction, so gas dissolution, which is difficult, and even impossible at higher temperatures with ozone, shifts the advantage in favor of singlet oxygen.
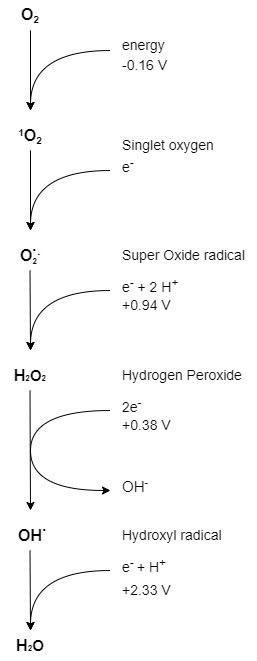
Figure 6. Reactive oxygen species derived from ambient oxygen
Let’s examine the singlet oxygen molecule and then discuss the implications of the difference between ozone and singlet oxygen as an ROS in industrial applications.
Three main techniques have been used to produce the singlet state of oxygen:
- excitation in the singlet state through ultraviolet irradiation in the presence of photosensitizers
- chelation with transition metals
- specific chemical reactions that result, under controlled conditions, in the emission of singlet oxygen
Singlet oxygen is short-lived in water as it interacts with other molecules by combining with them and transferring its excitation energy. The oxygen then returns to the triplet state, whereas the molecule that the oxygen has interacted with becomes excited. It has an oxidation potential of 2.42 mV, and together with hydroxyl radical •OH, at an oxidation potential of 2.8 mV, is one of the two most oxidative reactive oxygen species. The hydroxyl radical, the telltale sign of an advanced oxidation process (AOP), is the most powerful but shortest-lived ROS in water treatment, and it helps break down most chemical contaminants because it is a very non-selective oxidizer. Singlet oxygen reacts quickly with water to form hydroxy radicals, peroxide, and other ROS. In gas form, singlet oxygen is less aggressive toward sensitive metals, polymers, and elastomers, so, for example, while Teflon tubing is required for ozone, polyurethane is sufficient for singlet oxygen.
We understand that three different mechanisms activate oxygen:
Absorbing energy: When the oxygen molecule absorbs enough energy and the spin is reversed, this change stimulates the oxygen to react with organic molecules in a way it could not before.
Gaining an additional electron: Oxygen can gain an extra electron (i.e., it is reduced) to form superoxide, which in turn produces hydrogen peroxide.
Gaining an oxygen atom: Oxygen can gain an extra oxygen atom to form ozone (O3).
A New Force
Although various types of energy can be used to produce singlet oxygen and it has been more than 90 years since its discovery, efficient production in large quantities has not been made available in the industry. We report the first known large-scale production of singlet oxygen from the air as a practical solution in water treatment applications. Aquavative Technologies, Co., Ltd., a startup company based in Thailand, has innovated a hybrid photo/magneto-catalyst method to generate singlet oxygen from ambient air. Several pilot operations are underway, with a full range of applications requiring an effective AOP in the pipeline.
This new AOP is known as the Aquatron® process. It utilizes a proprietary hybrid magnetic/photonic nano-catalyst to affect the oxygen molecules in the incoming air and decrease the amount of photochemical energy necessary to convert it to singlet oxygen. While previous technologies have produced singlet oxygen in milligram (mg) quantities in the lab, now for the first time, large gram (g) or kilogram (Kg) quantities are available for industrial applications.
Singlet oxygen has several properties that make it interesting to apply in a broad range of applications. In cooling towers, hydroxyl radicals and singlet oxygen provide biocidal activity, biofilm removal, anti-scaling, and scale removal, as well as anti-corrosive properties. These applications include all areas that previously required the use of four or five different hazardous chemicals. When this new nano-catalytic process is coupled with a novel low-power electronic scale removal process, blowdown is no longer necessary. This saves water, and chiller efficiency is increased. Commercial applications for cooling towers have incorporated world-class Internet of Things (IoT)-based chiller efficiency and cooling tower (CT) efficiency monitoring, which is managed via Oracle’s advanced cloud-based management system, Oracle Cloud Infrastructure (OCI). Monitoring with thermal probes has an accuracy of less than 0.01°C uncertainty, which is about 10x the accuracy of most industrial thermal probes. When coupled with flow and kW measurements, chiller efficiency and load (kW/ Ton +/- 5 percent) can be recorded to the cloud in real time. This integrated system of data from the different devices and the applied analytics optimized management represents the first time the industry was able to get this kind of chiller load and efficiency monitoring that is low cost enough to use in almost any size system.
How Does This Innovation Compare To Other Technologies For ROS Generation?
Advanced oxidation processes have received close attention for the treatment of recalcitrant molecules that cannot be removed by traditional biological wastewater treatment processes. Legacy AOPs include techniques such as O3/UV, H2O2/UV, Fenton (Fe+3/ H2O2), etc. The O3/UV process requires extensive processing equipment to produce clean, dry, concentrated oxygen for supply to the ozone generator, which is notoriously picky and pesky to maintain. All the other AOPs require transport and the use of chemical inputs. Contrarily, hybrid magnetic/photonic devices produce industrial quantities of singlet oxygen from ambient air, and no chemical inputs are needed.
Large-scale industrial ozone generators require very clean, dry air (<-55°C dewpoint typically), which has the nitrogen removed in molecular sieves to obtain a very dry gas with about 90 to 95 percent oxygen, which feeds through a high voltage potential. Any moisture or other contaminants will result in sparks that destroy the generator’s dielectric coating. Any engineer who has maintained ozone generators in industrial settings is well aware of the difficulties in managing ozone generators. The equipment to prepare the air for ozone production can cost as much as or more than the ozone generator itself
Relative to the mineral oxychloride solution AOP reagent (MOCl), both AOPs have a very high yield of hydroxyl radicals. In contrast, MOCl employs chelation with transition minerals as the source of energy and can prolong and support the regeneration of (ROS) long after the point of activation without requiring external power at any point in the process. This self-catalytic continuation of AOP manifests a background chlorinated compound that accredits free chlorine residual as a viable method of monitoring mineral oxychloride implementations. When ambient air is catalyzed with magnetic/photonic energies, it produces singlet oxygen in the gas phase; however, with complete solubility in water, it will immediately convert to hydroxyl radicals. The mineral oxychloride AOP is implemented in the liquid phase from beginning to end. Both technologies support efficient monitoring with oxidation-reduction potential (ORP).
In addition to the obvious differences between magnetic forces and radiation requiring external energy source, and MOCl being a ready-to-use liquid chemical, we can say in simpler terms that both have comparable performances near the point of injection, while the mineral oxychloride treatment is at the same time more enduring, derived from a radical chain reaction that supports continuing regeneration of singlet oxygen and hydroxyl radicals. The continuing productivity of oxygen-based oxidants in water treated with MOCl is induced by the expendable oxidation energy from the mineral catalysts. This vibrational energy is readily available in solution, and when chlorine ions are present, they will form bonds with accessible dissolved oxygen and generate HOCl and OCl.
When considering which technology of generating singlet oxygen will be best suited for a particular application, one must examine the pros and cons of having prolonged oxidative reactivity supporting extended residual protection, along with the presence of chlorinated molecules supporting the option of free chlorine residual as monitoring control. Also important is evaluating whether a chemical OPEX is preferable, or if a larger CAPEX with lower running costs would be a better fit for your project.
Today, ozone is the most popular form of reactive oxygen sold worldwide, but singlet oxygen has many inherent advantages to ozone. Ozone’s difficulties in producing large quantities, requiring complex equipment and its lack of solubility, which further drops at higher temperatures, is a major issue. High-concentration ozone (5 to 20 percent) is required for most processes to reach high ORPs, but these high-concentration generators are costly to purchase and operate and require expensive peripheral equipment. In contrast, singlet oxygen’s reaction with water is an electrochemical process not affected by gas solubility. For these reasons, many customers are looking at new applications of this ROS, where ozone isn’t currently competitive. Finally, the reactions of hydroxyl radicals are more efficient for many organic molecules relative to ozone-based oxidation.
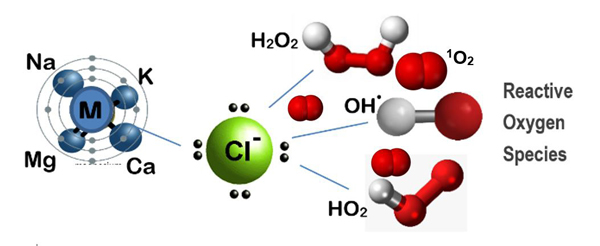
Figure 7. Proposed structures for mineral oxychloride advanced oxidation reagent, MxOyClz
What Else We Are Seeing
In shrimp hatcheries, an experiment was run on two influent water reservoirs, comparing traditional chlorination/thiosulphate deactivation with singlet oxygen treatment. Both reservoir sites showed complete eradication of thiosulfate-citrate-bile saltssucrose (TCBS) strain-based vibrio counts. However, a significant difference was found in the TCBS counts of water samples from the larval rearing tanks downstream. In the post-chlorination downstream tanks, TCBS counts were 2-3 x 104 CFU/ml, whereas larval rearing tanks using water from singlet oxygen treated reservoirs showed only 1-2 x 103 CFU/ml, and about 20x fewer vibrio bacteria caused higher shrimp survival rates and minimal deformities compared to traditional chlorination.
Power plants have shown significant reduction of fuel consumption as well as reduction of chemicals and water consumption after the new proprietary hybrid nano-catalyst equipment has been installed to treat their cooling water.
In drinking water, singlet oxygen allows for easy and effective removal of chemicals like endocrine-disrupting and pharmaceutical-active compounds, brominated hydrocarbons, and traces of personal care products, which can be very difficult to break down. It has been successfully implemented in an innovative bottled drinking water with reduced cluster size and the clients love that their water tastes very good and feels more refreshing. The singlet oxygen treatment allows for cost-effective removal of trace contaminants and optimization of the quality of the water.
In animal drinking water systems, singlet oxygen’s ability to not only disinfect with hydroxyl radicals, but its ability to attack biofilms in the water-distribution system make it an attractive option for broilers, layers, swine, and dairy operations.
 About The Authors
About The Authors
Daniel E. Gruenberg, is the Chairman & CTO of Aquavative Technologies, Co., Ltd., a Thailand-based startup with focus on designing, installing, and monitoring of innovative water treatment solutions with goals of saving water, reducing chemical use, and producing “negawatts” by promoting efficiency through continuous monitoring of systems and heat exchange processes. Email: daniel@aquavative.com
 Emma Flanagan is the CEO/CTO of Envirocleen, LLC, an Illinois water treatment chemical consulting company, manufacturer, and distributor of mineral oxychloride advanced oxidation reagent and quantum disinfection technologies. Email: emma@envirocleen.com
Emma Flanagan is the CEO/CTO of Envirocleen, LLC, an Illinois water treatment chemical consulting company, manufacturer, and distributor of mineral oxychloride advanced oxidation reagent and quantum disinfection technologies. Email: emma@envirocleen.com
