Ultrafiltration vs. Microfiltration
By Nick Nicholas
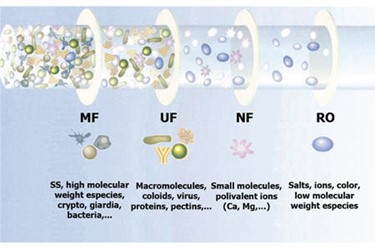
Which Process Should You Choose For Your Application?
Anyone who has studied at the very least high school level biology (and paid attention) is familiar with the concept of a membrane, in particular a semi-permeable membrane. Biological living cells are wrapped in semi-permeable membranes that keep their functions separate from the surrounding environment. The semi-permeable aspect allows only certain ions and small organic and other molecules to pass into or out of the cell. The membrane can be selective in either a passive or active capacity. Ultrafiltration (UF) and microfiltration (MF) processes utilize a semi-permeable membrane to separate microcontaminants from a water stream.
What is the difference between UF purification and microfiltration? We will first explain how a semi-permeable membrane works. Following this, we will explain the differences between the UF and microfiltration membrane treatment processes for water and wastewater treatment.
How does a semi-permeable membrane work?
One of the ways, in biological systems (i.e., living cell transport), is through active transportation across a membrane's surface, which occurs in a few different ways. Each one of these transports requires the cell to expend a certain amount of energy. One way is through transport channels that pull in and expel nutrients and metabolic wastes, respectively. Another is endocytosis, whereby the cell wall forms something like a pseudo-mouth, wrapping around an external object and then budding off within the cell as a vesicle. Its opposite is exocytosis — internal vesicles fuse with the membrane and its contents are secreted out into the surrounding solution.
Another way is through passive mechanisms that are known as diffusion and osmosis. Diffusion is the movement of ions and molecules from areas of high concentration to areas of low concentration in order to create a state of equilibrium on both sides of the membrane. As these ions move about, they create an osmotic pressure difference. Osmosis works opposite diffusion, seeking to create equilibrium by moving a solvent fluid (typically water) to the higher concentration area, although both work to achieve the equilibrium of the same concentration of solutes in so there is no osmotic pressure differential.
The passive diffusion/osmosis process is a mechanism that is easy to replicate on a much larger scale in non-biological systems. There are many potential applications for such technology, but it has particular usefulness in water and wastewater treatment. Micro- and ultrafiltration purification are two such membrane technologies.They are very similar filtration/separation processes with a difference that makes each ideal for their own particular applications.
Microfiltration and UF purification are more alike than they are different. As mentioned in the introduction, they are both non-biological (non-living), membrane-based separation technologies. These system processes work by applying differential pressure across a semi-permeable membrane and that pressure forces water, solutes, and small particulate matter through the membrane while larger solids are retained on the other side.
These processes both also make for beneficial pretreatment steps for reverse osmosis and nanofiltration (RO/NF). Membranes need to be deployed in properly designed systems and sometimes need periodic cleaning so they can last as long as possible without replacement. Filtration and precipitation pretreatment reduces concentrations of larger solid particles and reduce severity of membrane fouling.
The membranes for these microfiltration and UF purification systems are available in the same configurations. Plate and frame, tubular, hollow fiber, and spiral wound are possible options. These different configurations offer their own pros and cons. There are also different materials the membranes can be composed of, namely polymers, ceramic, and a few metal versions.
Similar Benefits:
-
No chemicals
-
Constant product quality regardless of feed quality
-
Easily scalable
Similar costs:
-
Equipment
-
tanks, pumps, skids, controls, etc.
-
-
Construction materials
-
Water characterization
-
What is in the water/wastewater determines what needs to be done to treat it properly. More complex compositions or high concentrations of pollutants will require pretreatment steps or more energy-intensive system design or more resilient membranes to handle these conditions. Low concentrations and simple contaminant compositions tend to require less pretreatment and therefore reduce operational costs..
-
-
Flow rates
-
Higher flow rates are associated with higher capital and operational costs
-
-
Planning
-
Space requirements
-
Installation
-
Prepackaged vs Unassembled systems
-
-
Shipping fees
-
Operational costs
The Differences
It all boils down to pore size. On the membrane separation scale, micro- and ultrafiltration are coarser than nanofiltration (NF) and reverse osmosis (RO), but are still finer than media filtration. Although there is not universal agreement, commonly used definitons have microfilter pores within the range of 0.1 to 10 microns and ultrafiltration membrane pores within 0.005 to 0.1 microns. Ultrafiltration membranes are typically rated by the molecular weight of the solids they remove — and are typically rated from 5000 to 300,000 molecular weight cut-off. The membrane selected for a treatment system is based on the size of the smallest particles to be retained in the feedwater. The difference in their pore size determines the applications for which ultrafiltration purification or microfiltration treatment process would be the most applicable to be applied for the specific application.
Removal
Based on the pore size range of these two separation technologies, below is a list of some of the smallest pollutants that each technology is capable of removing or reducing from raw water streams.
Microfiltration
-
Algae
-
Bacteria
-
Pathogenic protozoa (Giardia lamblia and Crypotosporidium)
-
Sediment (sand, clay, certain complex metals/particles)
Ultrafiltration
All of the contaminants MF can remove plus:
-
Endotoxins
-
Colloids (including colloidal silica)
-
Silt
-
Viruses
-
Proteins
Applications
Both microfiltration and UF purification are useful for water/wastewater treatment in a broad range of industrial and commercial settings. This includes the processing of many kinds of end products. Below are only a few of the many possible applications for each membrane filter process.
Microfiltration
-
Cold sterilization of beverages and pharmaceuticals
-
Separating bacteria from water, liquid pharmaceuticals, and medicines
-
Clarifying fruit juices, wine, or beer
-
Petroleum refining
-
Silt density index reduction for reverse osmosis
-
Virus removal from water
-
Separating oil/water emulsions
-
Removing pathogens from milk
-
Medical applications
-
Foodstuff fractionations
