Enhancing Clean-In-Place (CIP) Systems With Pulsafeeder's NextStep Pump Technology
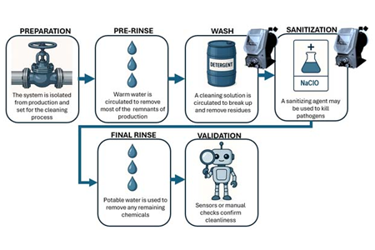
Clean-in-Place (CIP) systems are critical for industries where hygiene and contamination control are mandatory—such as food and beverage, pharmaceuticals, and specialty chemical manufacturing. These systems automate cleaning through validated, programmed steps that ensure compliance with stringent safety and quality standards. This white paper explores the challenges of CIP operations and demonstrates how Pulsafeeder’s NextStep pump technology addresses these challenges with precision, reliability, and cost efficiency.
Before the advent of automated cleaning systems, operators manually cleaned production equipment between batches—a time and labor-intensive process prone to human error. Inadequate cleaning could lead to cross-contamination, bacterial growth, and severe health risks, including foodborne illnesses and product contamination. To mitigate these risks, regulatory bodies such as the U.S. Food and Drug Administration (FDA) enforce strict standards under the Food Safety Modernization Act (FSMA) and Current Good Manufacturing Practices (CGMP). These regulations mandate comprehensive food safety plans and validated cleaning protocols to prevent contamination and ensure consumer safety.
Get unlimited access to:
Enter your credentials below to log in. Not yet a member of Water Online? Subscribe today.
