The Nationwide Impact Of California's Hexavalent Chromium Regulations
By Dr. Philip Brandhuber, professional associate, HDR Engineering
California recently became the first U.S. state to regulate hexavalent chromium in drinking water. Will others follow suit?
On July 1, 2014, California became the first state in the nation to regulate hexavalent chromium — Cr(VI) — in drinking water. The California Department of Public Health set a maximum contaminant level (MCL) of 0.010 mg/L (10 μg/L) for Cr(VI). This regulation is in addition to the existing California unique MCL of 0.050 mg/L for total chromium — Cr(tot). Total chromium is defined as the sum of the trivalent chromium — Cr(III) — and Cr(VI) species. Outside of California, Cr(VI) remains unregulated, but non-California drinking water utilities must meet the less stringent Cr(tot) MCL of 0.100 mg/L established by the U.S. EPA. The EPA has not announced if it will introduce a national Cr(VI) regulation, but it is likely that a Cr(VI) regulation will be promulgated in the future. The purpose of this article is to familiarize readers with the possible regulation and treatment of Cr(VI) in drinking water.
Chemistry And Sources Of Chromium
Chromium is the earth’s 21st most abundant element. It can enter water through the erosion or dissolution of chromium-bearing rocks or minerals as well as through man-made contamination. In natural water, chromium exists in two oxidation states. The oxidized species, Cr(VI), is a negatively charged ion and highly soluble at the pH range of drinking water. The chemistry of the reduced species, Cr(III), is more complex. Depending on pH, it can be a negatively charged ion, an uncharged molecule, or a positively charged ion. At moderate pH (7-9), Cr(III) forms an insoluble hydroxide. Soluble Cr(III) can also associate with organic matter or metal oxides. Hence, while Cr(VI) is quite soluble in water, a large portion of Cr(III) in water may be in particulate form. In natural systems, the presence of manganese-bearing minerals can oxidize Cr(III) to Cr(VI), while the presence of natural organic matter tends to reduce Cr(VI) to Cr(III).
Strong oxidants, like free chlorine, commonly used by utilities for disinfection, can affect chromium speciation by oxidizing Cr(III) to Cr(VI). The kinetics (speed) of the reaction is relatively slow under conditions seen in drinking water plants, but the reaction may become important in distribution systems where Cr(III) can have several days to oxidize to Cr(VI) under the influence of the residual disinfectant. Understanding the extent of this problem is the subject of current research.
The national occurrence of chromium in drinking water is being studied. A 2004 investigation by the Water Research Foundation (WRF) of 400 utilities found an average raw water Cr(VI) concentration of 1.1 μg/L. In anticipation of a possible regulation of Cr(VI), the EPA is requiring utilities to monitor Cr(tot) and Cr(VI) in their treated water as part of the Third Unregulated Contaminants Monitoring Rule (UCMR3). The monitoring period started in January 2013 and will close in December 2015. Through July 2014, the EPA reports that of 2,640 monitored systems, 66 percent detected Cr(tot) (reporting level = 0.2 μg/L), and 87.6 percent detected Cr(VI) (reporting level = 0.03 μg/L). The reader should note that these reporting levels are far lower than the California Cr(VI) MCL. If the EPA establishes a national Cr(VI) MCL similar to California’s, it is estimated that 2 to 4 percent of all drinking water systems will be impacted.
Chromium’s Health Effects
The movie Erin Brockovich heightened the public’s sensitivity to chromium, but the movie did not accurately reflect chromium’s risks. Trivalent chromium is not harmful to humans at low concentrations. In fact, it is a micronutrient necessary for good health. The EPA classifies Cr(VI) as a carcinogen via exposure by inhalation, but the adverse health effects of low level Cr(VI) exposure via ingestion are less clear. Much of the debate about Cr(VI)’s human health risk centers on how effectively ingested Cr(VI) is detoxified by the gastrointestinal tract. At present, the EPA has not finalized its findings regarding the health risks of Cr(VI) via ingestion, although the State of California independently concluded that ingestion of Cr(VI) in water at levels greater than 10 μg/L represents an unacceptable public health risk.
Detection Of Chromium In Drinking Water
Chromium can be accurately measured to sub-part per billion concentrations using widely available analytical techniques. Total chromium concentrations are determined by inductively coupled plasma — mass spectrometry (ICP-MS) per EPA Method 200.8. Hexavalent chromium is measured by ion chromatography followed by spectroscopic detection per EPA Method 218.7. There is no accepted method for direct measurement of Cr(III). Instead, Cr(III) concentrations are calculated by difference, subtracting the Cr(VI) concentration from the Cr(tot) concentration.
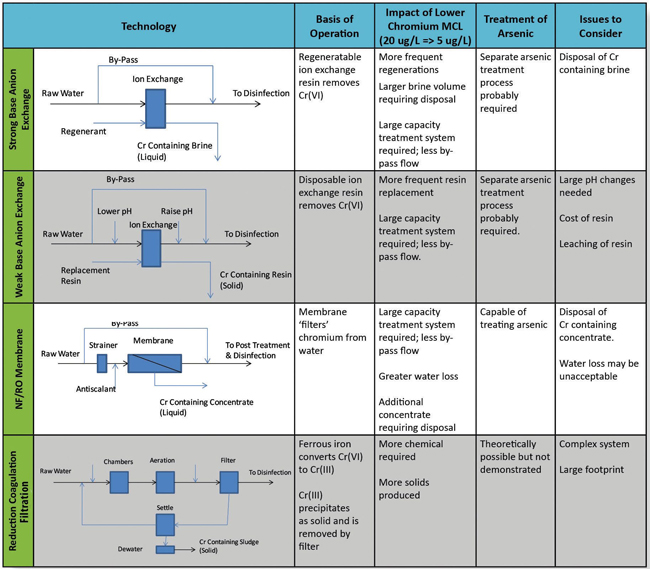
Technologies For The Treatment Of Chromium In Drinking Water
Currently few utilities in the U.S. operate a full-scale treatment system specifically designed to remove Cr(VI) from drinking water. However, there have been a number of bench and pilot level studies evaluating chromium treatment technologies. Based on the results of these studies, there are four Cr(VI) treatment technologies capable of meeting California’s 10 μg/L MCL:
- Strong base anion exchange (SBA)
- Weak base anion exchange (WBA)
- Nanofiltration/reverse osmosis membranes (NF/RO)
- Reduction/coagulation/filtration (RCF).
NF/RO membranes remove Cr(VI) by forcing contaminated water under pressure through a semipermeable membrane. Hexavalent chromium, along with other dissolved contaminants, is retained by the membrane through a combination of size exclusion and electrostatic repulsion effects. Depending on the specific design of the membrane, rejection of up to 95 percent of Cr(VI) is possible. As with all NF/RO systems, they are hydraulically inefficient. A typical recovery (the ratio of water produced to water treated) is 75 to 80 percent. Hence, membrane systems are undesirable in arid sections of the country where water resources are scarce. In addition, disposal of the residual (waste) stream, in which most contaminants are concentrated to four to five times their background level, is often problematic.
SBA exchange removes Cr(VI) by passing the contaminated water through a bed of polymeric resin with chloride ions attached to charged functional groups integrated into the resin. The negatively charged Cr(VI) ions displace the chloride ions, attaching the Cr(VI) ions to the resin while releasing chloride ions into the treated water. Once the resin is exhausted, and there are no additional sites on the resin to take up Cr(VI), the resin is regenerated with a high concentration sodium chloride solution. The chloride ions reattach to the resin, and Cr(VI) is released into the salt solution for disposal. A similar process is used to treat arsenic and nitrate in drinking water. Pilot testing has found that SBA is more effective in removing Cr(VI) and less sensitive to the presence of co-occurring ions, like sulfate, than when used for arsenic or nitrate treatment. Several thousand bed volumes of throughput may be obtained when treating Cr(VI) before regeneration is needed. Multiple regenerations with the same sodium chloride solution are also possible. Hydraulically, SBA is very efficient, obtaining greater than 99 percent recovery. The waste stream produced by SBA is classified as a hazardous liquid waste. It consists of a highly concentrated saline solution containing Cr(VI) and other ions removed by the SBA process. Additional processing of the residuals stream is required if a hazardous liquid classification is to be avoided.
WBA exchange also removes Cr(VI) by passing the contaminated water through a bed of polymeric resin. Unlike SBA, the WBA technology is not regenerated and operates as a single-use disposable medium. Throughputs of well over 100,000 bed volumes have been demonstrated by pilot tests. The term ion exchange is somewhat of a misnomer for this technology, since the throughput far exceeds the ion exchange capacity of the resin. In fact, it appears Cr(VI) is removed by reduction and precipitation of chromium on the resin. An important drawback of this technology is its sensitivity to pH. To be effective, the technology must operate at pH 6 or less. Generally, the pH of the water must be depressed for treatment and then raised to produce a non-corrosive stable water for distribution. Given the quality of most drinking water sources, substantial quantities of chemicals are needed to make these adjustments. Operating as a single-use disposable medium greatly simplifies problems with disposing of the treatment residuals, but this advantage is off-set by the technology’s need for chemical handling facilities along with its considerable chemical consumption.
RCF is a multi-step treatment process in which Cr(VI) is converted to Cr(III), and the Cr(III) is then removed by filtration. Specifically, ferrous iron (Fe(II)) in the form of ferrous sulfate or ferrous chloride is used to reduce Cr(VI) to Cr(III). At moderate pH, the Cr(III) precipitates as chromium hydroxide, Cr(OH)3 by the following partial reaction:
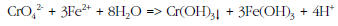
A coagulant is used to aggregate Cr(III) into flocs suitable for removal via sedimentation followed by filtration with deep bed media or low-pressure membranes. The RCF process is widely used for industrial chromium treatment and is similar to the conventional treatment process used by many drinking water utilities. Hence, drinking water utilities are quite familiar with the basic design concepts, equipment, and chemicals used by this process. The RCF process produces a non-hazardous residuals stream that can be handled and disposed of in the same manner as residuals from a conventional treatment plant. However, the RCF technology uses multiple unit processes and chemical feeds that by necessity take up a large footprint. The RCF process also requires a good deal of operator attention. For these reasons, the technology is better suited for surface water systems, which typically treat a small number of sources at a central location, rather than groundwater systems, which typically consist of multiple, widely distributed wells located on small sites.
Looking Ahead
The State of California has established an MCL for Cr(VI) in drinking water of 0.010 mg/L. The MCL applies only to California, and there is no national MCL for Cr(VI). The U.S. EPA is in the process of determining if a national Cr(VI) regulation is justified. No date has been set by the U.S. EPA to announce a regulatory determination for Cr(VI). If Cr(VI) is regulated by the U.S. EPA at a level similar to California’s, about 2 to 4 percent of all utilities nationally will be impacted. While the drinking water industry has little experience in treating Cr(VI), a number of studies have been performed investigating Cr(VI) treatability. Four technologies, NF/RO, SBA exchange, WBA exchange, and RCF, are effective for controlling Cr(VI) to the California MCL. The most suitable technology for any individual treatment situation will vary, but it is greatly influenced by the disposal scenario for the treatment residual.

Dr. Philip Brandhuber is a professional associate at HDR Engineering in Denver, CO. Brandhuber is HDR’s expert in the treatment of inorganic contaminants in drinking water using advanced technologies. He can be reached at Philip.Brandhuber@hdrinc.com.
