Taking The "Forever" Out Of PFAS: The Future Of PFAS Remediation
By Tamzen Macbeth and Charles Schaefer

As PFAS treatment technologies continue to emerge, CDM Smith reviews some considerations for the existing options — and introduces a new one.
Per- and polyfluoroalkyl substances (PFAS) are some of the most difficult chemicals to break down, or destroy, due to the strength of the carbon-fluorine bond, the strongest bond in chemistry. Most PFAS destructive technologies require extreme temperature or pressure, caustic conditions, or harsh chemical additives and consume tremendous amounts of energy.
Currently, no destructive technology has been demonstrated at full scale for large volumes of contaminated water. In addition, drinking water providers have relied on conventional technologies using sorption, ion exchange, or sequestration to separate and concentrate PFAS into other media or waste streams. However, this poses the risk of re-releasing these “forever” chemicals back into the environment. As such, destruction is a critical step in solving the global PFAS crisis.
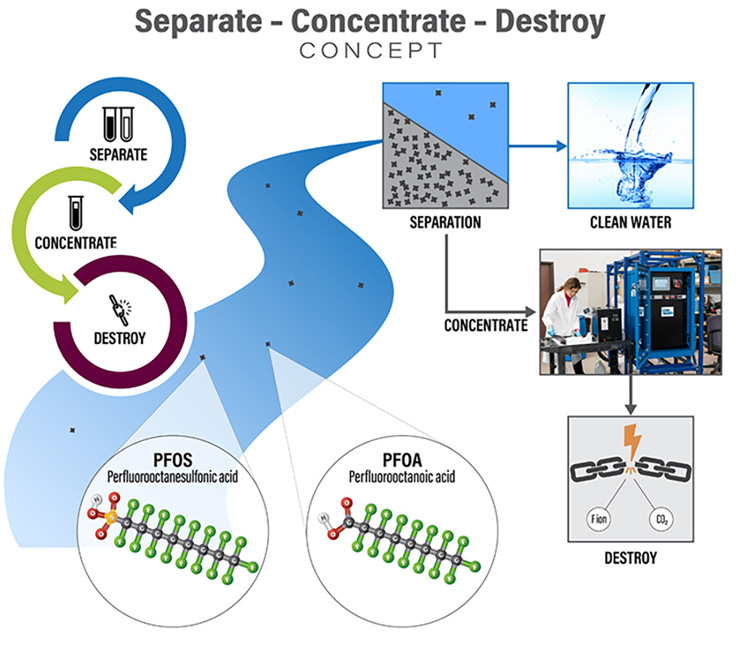
Today, destruction technologies such as electrochemical oxidation (ECO), plasma, and supercritical water oxidation have demonstrated abilities to break down PFAS. To integrate these promising technologies for future water treatment, treatment trains that first separate and concentrate PFAS to reduce the volume are necessary to make these (and other) destructive treatments viable.
Separate And Concentrate
To address urgent needs to remove PFAS from drinking water supplies, we have developed methods to evaluate, design, and implement at full scale reliable technologies such as granular activated carbon (GAC), ion exchange (IX), and reverse osmosis (RO) for PFAS treatment. However, these technologies still generate PFAS-laden waste streams with volumes that are often impractical to treat with destructive technologies. Our researchers have been rigorously testing new ways to separate and concentrate PFAS that can be used along with or in place of these conventional treatments.
One particularly promising technology relies on using air bubbles to “strip” PFAS out of water and into foams, which are condensed to highly concentrated PFAS solutions. This technology has achieved concentration factors of 90,000 times at full scale and ongoing optimizations are working to achieve concentration factors of one million times. In other words, one million gallons of PFAS could be treated generating approximately one to 10 gallons of PFAS concentrate for destructive treatment. Together with EPOC Enviro, CDM Smith has been rigorously testing surface-active foam fractionation (SAFF®) in our treatment train concept. We recently completed the first U.S. pilot application, successfully treating 265,000 gallons of PFAS-contaminated groundwater and generating three gallons of PFAS concentrate. The concentrate has been sent to our laboratory in Denver, where it is being treated with our pilot destructive ECO system.
Destroy
Numerous PFAS destruction technologies are under development (see https://pfas-1.itrcweb.org/12-treatment-technologies/). Promising destruction technologies that have progressed from bench- to pilot-scale include ECO, plasma, UV-reductive, hydrothermal, and supercritical water oxidation. These technologies have successfully treated an array of water samples highly concentrated with PFAS and are considered ideal for destructive treatment, including one or more of the following:
- Aqueous film-forming foam (AFFF) concentrates
- Groundwater within PFAS source areas
- Remediation waste streams (such as wastewater generated from regeneration of GAC or regenerable IX resin; foam-fractionation, soil-washing, rejected-RO concentrates; chemical-or electro-coagulation.
- Landfill or biosolid leachate
Electrochemical Oxidation (ECO)
Our researchers have proven ECO to reduce high-concentration PFAS effectively, typically achieving reductions of 90% to 99.999% in laboratory and pilot studies.
ECO uses an electrochemical cell to generate an electric current between a reactive anode and cathode (the electrodes). The process degrades PFAS through two mechanisms:
- Anodic oxidation (direct electrolysis) – PFAS adsorb onto an anode surface and are destroyed directly at the electrode by a direct electron transfer reaction.
- Indirect oxidation – Strong oxidizing and nonselective radicals (such as hydroxyl, oxygen, sulfate, and carbonate) are generated in situ that react with, and break down, PFAS in the bulk liquid reactions.
Choosing A Destruction Approach
Because of the high demand for destructive PFAS technologies, they are often promoted hastily without demonstrating complete destruction (e.g., defluorination) and without confidence the technology can meet stringent effluent discharge requirements. The feasibility of these technologies must be carefully considered for each new application. To effectively develop a treatment train approach, technology compatibility, engineering constraints, and O&M requirements must be considered.
Currently, technology evaluation for a particular site/application must include bench- and pilot-scale tests to demonstrate technology and incorporate economic feasibility in the selection process. A thoughtful approach will ensure the system can meet required treatment volumes, rates, and discharge criteria.
The development and commercialization of PFAS destruction technologies is in its infancy. Technology benefits and limitations should be discussed with technology providers, including:
- Energy demand and efficiency to achieve desired treatment goals at the scale required for the system.
- Health and safety concerns.
- O&M requirements and longevity of the system.
- Scale of available systems and feasibility of operating largescale systems, if required.
- Potential for incomplete PFAS destruction resulting in accumulation of fluorinated intermediates that are generated but not measurable.
- Feasibility of achieving stringent (i.e., very low) treatment requirements. Often a treatment train approach may be needed before effluent discharge.
- Effectiveness in destroying all PFAS chemicals, including short-chain PFAS (which are generally harder to treat) and precursors as sources of perfluoroalkyl acids (PFAAs).
- Generation of non-PFAS toxic byproducts, such as perchlorate or hydrofluoric acid. For instance, perchlorate is known to be formed during electrochemical oxidation treatment due to the aggressive oxidation of chloride in the feedwater. Although perchlorate can be addressed easily, treatment systems must account for, and treat, perchlorate in the process
CDM Smith has been investigating PFAS destruction for nearly a decade. Our approach to assessing PFAS destructive technologies at a site includes treatability testing at the bench-, pilot-, and full-scale levels, using three lines of evidence to confirm complete PFAS destruction.
 About The Authors
About The Authors
Tamzen Macbeth, PhD, PE, BCEE, is an internationally recognized remediation expert who develops innovative, cost-effective technologies for contaminated soil, sediment, and groundwater. Tamzen has helped advance countless technologies within her field, publishing more than 100 technical papers, training manuals, and guidance documents on remediation topics.
 Charles Schaefer Jr., PhD, is an environmental scientist and the director of CDM Smith’s Bellevue, Washington, Research and Testing Laboratory. Charles has received multiple awards for his research into PFAS, most recently earning a top prize from the American Society of Civil Engineers (ASCE) for investigating AFFF with the U.S. Department of Defense.
Charles Schaefer Jr., PhD, is an environmental scientist and the director of CDM Smith’s Bellevue, Washington, Research and Testing Laboratory. Charles has received multiple awards for his research into PFAS, most recently earning a top prize from the American Society of Civil Engineers (ASCE) for investigating AFFF with the U.S. Department of Defense.
