Generating Disinfectants From Water: Could In Situ Electrolytic Production Be The Future?
By Dr. Samuel Stucki, Scientific Consultant to Innovatec Gerätetechnik GmbH,Germany
Chemical disinfection using strong oxidants involves the transportation, storage, and handling of unstable and/or aggressive and hazardous chemicals. The production of oxidants at the location where they are to be used has therefore been a preferred option, especially in small-scale or remote installations. On-site electrolysis of NaCl for the local production of chlorine or hypochlorite, or the production of ozone-enriched oxygen by silent discharge has been standard for years. However, it is possible to take the idea of producing chemical disinfectants locally a step further and look at in situ techniques, i.e. at devices, which produce oxidants in and from the water to be treated.
In situ techniques offer a number of advantages as they can be introduced into the water systems where needed. They do not require extra equipment for contacting and the dosage can be controlled very easily by the electric input into the device. An example of such a promising new technology is a miniaturized low-cost electrochemical ozone generator that can be integrated very easily into distribution piping (Fig. 1).
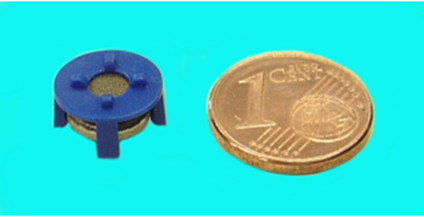
Fig. 1: Miniaturized electrochemical cell (compared to a one Euro cent coin) producing ozone from tap water
The Process
Water can be the source of a number of more-or-less short-lived oxidants ranging from the extremely unstable and reactive hydroxyl (HO) and hydroperoxyl (HOO) radicals to more common disinfectants such as ozone or hydrogen peroxide, and eventually molecular oxygen. One important property of water-derived disinfectants is that they decompose to harmless species, i.e. water or molecular oxygen. This means the formation of potentially hazardous secondary products can be minimized.
The production of oxidants from water requires that the energy for splitting water molecules be overcome. For this to work, either electricity or vacuum UV radiation (VUV, wavelength <185 nm) can be used. Electric discharge technologies that apply high voltages directly to the water have been studied in the laboratory; however it is not clear whether they can be made efficient, reliable and safe enough for widespread application. VUV can split water to produce hydroxyl (HO) and hydroperoxyl (HOO) radicals. This technology has been used to oxidize trace TOC, with the main application in TOC monitors for ultrapure water systems. The radicals produced by VUV-photolysis of water are very short-lived and have no practical importance for disinfection purposes. In comparison, electrochemical processes operating at low voltages of a few volts dc seem more appropriate for decentralized applications and have been shown to be tunable to produce the full range of oxidants from water.
An electrochemical cell for the electrolysis of water consists of two electrodes (anode and cathode) and a strong aqueous electrolyte that does not decompose in the process. Feeding a dc current to such a cell yields the products of water splitting, i.e. hydrogen, at the reducing cathode, and oxygen at the oxidizing anode in stoichiometric quantities proportional to the electric charge that passes through the electrodes. The oxidation process at the anode that ultimately leads to the evolution of oxygen gas proceeds via a number of reactive intermediate oxygen species. Which intermediates are formed with what yield depends on the surface properties of the anode material. It has been long known that, by using specific electrolytes, electro-catalytic anode materials, and appropriate process conditions, fractions of up to 50 percent of the oxygen can be produced in the form of highly reactive ozone. As the conductivity of water as an electrolyte is a limiting factor in the operation of in situ electrolysis cells, practical applications have used a chemically inert polymer electrolyte membrane (PEM), which exhibits a high ionic conductivity and allows the operation of cells with high current densities and hence high production rates (Fig. 2).
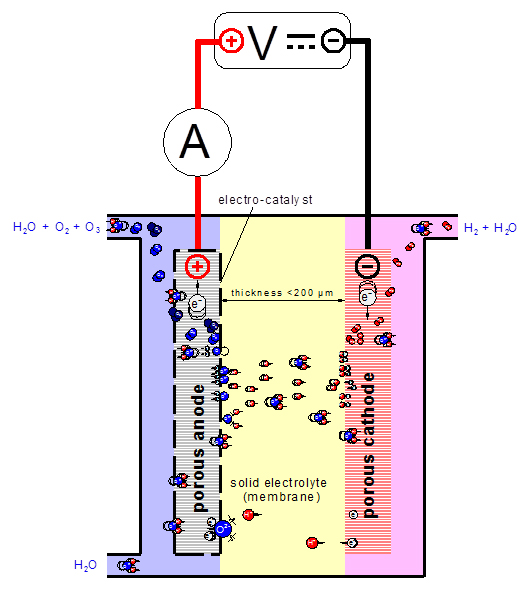
Fig. 2: Water electrolysis process in a PEM cell.
The use of ozone-generating anode materials in an electrochemical cell using PEM as an electrolyte was first described more than 30 years ago and has proved to be the most interesting option for producing ozone in situ from water.
Anodes for the production of ozone consist of corrosion resistant metal structures with coatings of β-PbO2, doped SnO2 or, more recently, thin films of semiconducting diamond. While β-PbO2 anodes only produce mixtures of ozone and oxygen, the primary product of doped diamond are OH radicals, which can then either react directly with substrates or recombine to form H2O2 and/or ozone.
Application of ozone generating PEM electrolysis cells
Ozone-generating PEM cells were originally developed for the efficient disinfection of industrial ultrapure water systems. Since they can be operated to maintain a very low (<10 ppb) background concentration of ozone in the system, they are very suitable for keeping water systems sterile. Typically an ultrapure water loop of some 20m3/h capacity can be kept clean with an electrolysis device with 250 W power input. In order to guarantee the absence of ozone in the water in the pharmaceutical production process, UV lamps are situated at the point of use to destroy residual ozone. In situ ozonation has been the state-of-the-art world-wide for more than 20 years now, replacing the much more energy intensive hot storage technology still in use in many pharmaceutical plants, especially in the US. The set-up of an ozone generating electrolysis cell in a typical application to keep an ultra-pure water loop in a pharmaceutical production plant sterile is shown in Fig. 3.

Fig. 3: Ultra-pure water sterilizing unit, complete with electrochemical ozone generator, power supply unit, pumps, uv lamp for ozone removal and tubing.
(Photo courtesy BWT Group).
The in situ production of ozone by electrolysis using a PEM cell configuration offers a simple and easy to operate alternative to the established technology of ozone production, which includes silent discharge in oxygen gas and contacting the ozone-enriched gas with the water. So far, however, commercially significant applications of in situ electrolysis for the production of ozone have been limited to ultrapure water systems. There are two main reasons why in situ electrolytic production has not been applied in drinking water systems:
- The electrolysis process for ozone production is energetically less efficient than the production by silent discharge in oxygen or air. This limits the application to relatively small scales, where process advantages are more important than specific energy consumption;
- Electrolysis in the presence of Ca and Mg ions in water leads to the formation of scale on the cathode and results in cell failure for most designs of cells. Removing Ca and Mg by ion exchange softening is the straightforward solution to the problem of scaling. However, the extra process stage requires maintenance and compromises the simplicity of an in situ device without pre-treatment.
Developing the potential of PEM ozone generators for drinking water disinfection
Promising R&D is currently being carried out to demonstrate the feasibility of scaled-down PEM ozone generators. Such ozone microcells (see fig. 4) can be designed to be much less affected by water hardness and have been shown to operate for thousands of hours in natural water containing scale-forming cations.

Fig. 4: Stack of two miniaturized PEM electrolysis cells (Microcell®) for direct application in tap water. The PEM cell consists of porous electrodes and a membrane disk assembled in a blue plastic clip and mounted on a ¾” thread socket, which can be fitted to standard water tubing or containers. Power is supplied via a standard dc connector at the bottom of the socket.
The heart of the cell is a simple expendable part containing porous electrodes of 0.2 cm2 of active area and the membrane electrolyte. The cell heart is clamped onto a holder socket that fits into standard piping and provides a connector to the dc power supply. The cell heart can be replaced very easily. DC power of 5 to 12 Volts at up to 300 mA is supplied by the mass-produced power adapters, which are in wide use for charging battery-operated electronic gadgets. These features make the technology low-cost and well suited for application at the end of the water supply chain, i.e. in homes, or in water systems that are not connected to a public distribution network. The production capacity of the microcell is minute (a few mg of ozone per hour). However, given the low cost of the equipment, many of such cells can be installed in a water piping system at the very locations where the control of growth of microorganisms is crucial. As a result, control of microbiological contamination in local water distribution can be carried out very efficiently and with an optimized minimum of disinfectant by adjusting the ozone production to the demand at a given location in the system.
